Abstract
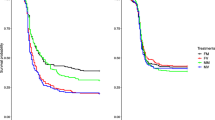
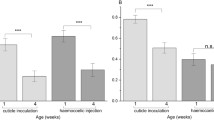
Susceptibility to pathogens and genetic variation in disease resistance is assumed to persist in nature because of the high costs of immunity. Within immunity there are different kinds of costs. Costs of immunological deployment, the costs of mounting an immune response, are measured as a change in fitness following immunological challenge. Maintenance costs of immunity are associated with investments of resources into the infrastructure of an immune system and keeping the system at a given level of readiness in the absence of infection. To demonstrate the costs of immunological maintenance in the absence of infection is considered more difficult. In the present study we examined the maintenance costs of the immune system in lines of Drosophila melanogaster that differed in their antibacterial innate immune response under starved and non-starved conditions. Immunodeficient mutant flies that have to invest less in the immunological maintenance were found to live longer under starvation than wild type flies, whereas the opposite was found when food was provided ad libitum. Our study provides evidence for the physiological cost of immunological maintenance and highlights the importance of environmental variation in the study of evolutionary trade-offs.


Similar content being viewed by others
Explore related subjects
Discover the latest articles and news from researchers in related subjects, suggested using machine learning.References
Dushay, M. S., Asling, B., & Hultmark, D. (1996). Origins of immunity: Relish, a compound Rel-like gene in the antibacterial defense of Drosophila. Proceedings of the National Academy of Sciences of the United States of America, 93(19), 10343–10347.
Fellowes, M. D. E., Kraaijeveld, A. R., & Godfray, H. C. J. (1998). Trade-off associated with selection for increased ability to resist parasitoid attack in Drosophila melanogaster. Proceedings of the Royal Society B, 265(1405), 1553–1558.
Haine, E. R., Moret, Y., Siva-Jothy, M. T., & Rolff, J. (2008). Antibacterial defense and persistent infection in insects. Science, 322, 1257–1259.
Harbison, S. T., Yamamoto, A. H., Fanara, J. J., Norga, K. K., & Mackay, T. F. C. (2004). Quantitative trait loci affecting starvation resistance in Drosophila melanogaster. Genetics, 166(4), 1807–1823.
Hedengren, M., Åsling, B., Dushay, M. S., Ando, I., Ekengren, S., Wihlborg, M., et al. (1999). Relish, a central factor in the control of humoral but not cellular immunity in Drosophila. Molecular Cell, 4(5), 827–837.
Hoang, A. (2001). Immune response to parasitism reduces resistance of Drosophila melanogaster to desiccation and starvation. Evolution, 55(11), 2353–2358.
Hoffmann, J. A. (1995). Innate immunity of insects. Current Opinion in Immunology, 7, 4–10.
Hoffmann, A. A., Hallas, R., Sinclair, C., & Mitrovski, P. (2001). Levels of variation in stress resistance in Drosophila among strains, local populations, and geographic regions; patterns for desiccasion, starvation, cold resistance, and associated traits. Evolution, 55(8), 1621–1630.
Hoffmann, A. A., & Harshman, L. G. (1999). Desiccation and starvation resistance in Drosophila: Patterns of variation at the species, population and intrapopulation levels. Heredity, 83, 637–643.
Hoffmann, A. A., & Parsons, P. A. (1989). An integrated approach to environmental stress tolerance and life-history variation: Desiccation tolerance in Drosophila. Biological Journal of the Linnean Society, 37(1–2), 117–136.
Hoffmann, A. A., & Parsons, P. A. (1993). Direct and correlated responses to selection for desiccation resistance: A comparison of Drosophila melanogaster and D. simulans. Journal of Evolutionary Biology, 6(5), 643–657.
Kraaijeveld, A. R., & Godfray, H. C. J. (1997). Trade-off between parasitoid resistance and larval competitive ability in Drosophila melanogaster. Nature, 389, 278–280.
Kraaijeveld, A. R., Limentani, E. C., & Godfray, H. C. J. (2001). Basis of the trade-off between parasitoid resistance and larval competitive ability in Drosophila melanogaster. Proceedings of the Royal Society B, 268, 259–261.
Leclerc, V., & Reichhart, J. M. (2004). The immune response of Drosophila melanogaster. Immunological Reviews, 198(1), 59–71.
Lemaitre, B., Kromer-Metzger, E., Michaut, L., Nicolas, E., Meister, M., Georgel, P., et al. (1995). A recessive mutation, immune deficiency (imd), defines two distinct control pathways in the Drosophila host defense. Proceedings of the National Academy of Sciences of the United States of America, 92(21), 9465–9469.
Libert, S., Yufang, C., Xiaowen, C., & Pletcher, S. D. (2006). Trade-offs between longevity and pathogen resistance in Drosophila melanogaster are mediated by NFκB signaling. Aging Cell, 5, 533–543.
Lochmiller, R. L., & Deerenberg, C. (2000). Trade-offs in evolutionary immunology: Just what is the cost of immunity? Oikos, 88, 87–98.
Luong, L. T., & Polak, M. (2007). Costs of resistance in the Drosophila–macrocheles system: A negative genetic correlation between ectoparasite resistance and reproduction. Evolution, 61(6), 1391–1402.
Matova, N., & Anderson, K. V. (2006). Rel/NF-κB double mutants reveal that cellular immunity is central to Drosophila host defense. Proceedings of the National Academy of Sciences of the United States of America, 103(44), 16426–16429.
McKean, K. A., Yourth, C. P., Lazzaro, B. P., & Clark, A. G. (2008). The evolutionary costs of immunological maintenance and deployment. BMC Evolutionary Biology, 8, 76.
Moret, Y., & Schmid-Hempel, P. (2000). Survival for immunity: The price of immune system activation for bumblebee workers. Science, 290(5494), 1166–1168.
Park, J. M., Brady, H., Ruocco, M. G., Sun, H., Williams, D. A., Lee, S. J., et al. (2004). Targeting of TAK1 by the NF-κB protein Relish regulates the JNK-mediated immune response in Drosophila. Genes and Development, 18(5), 584–594.
Parkash, R., & Munjal, A. K. (1999). Climatic selection of starvation and desiccation resistance in populations of some tropical Drosophilids. Journal of Zoological Systematics and Evolutionary Research, 37(4), 195–202.
Price, B. D., & Laughon, A. (1993). The isolation and characterization of a Drosophila gene encoding a putative NAD-dependent methylenetetrahydrofolate dehydrogenase-methenyltetrahydrofolate cyclohydrolase. Biochimica et Biophysica Acta, 1173(1), 94–98.
Råberg, L., Vestberg, M., Hasselquist, D., Holmdahl, R., Svensson, E., & Nilsson, J. Å. (2002). Basal metabolic rate and the evolution of the adaptive immune system. Proceedings of the Royal Society B, 269(1493), 817–821.
Roff, D. A. (2002). Life history evolution. Sunderland, MA: Sinauer Associates.
Royet, J., Reichhart, J. M., & Hoffmann, J. A. (2005). Sensing and signaling during infection in Drosophila. Current Opinion in Immunology, 17, 11–17.
Schmid-Hempel, P. (2003). Variation in immune defence as a question of evolutionary ecology. Proceedings of the Royal Society B, 270(1513), 357–366.
Sgrò, C. M., & Hoffmann, A. A. (2004). Genetic correlations, tradeoffs and environmental variation. Heredity, 93, 241–248.
Sheldon, B. C., & Verhulst, S. (1996). Ecological immunology: Costly parasite defences and trade-offs in evolutionary ecology. Trends in Ecology & Evolution, 11(8), 317–321.
Siva-Jothy, M. T., Moret, Y., & Rolff, J. (2005). Insect immunity: An evolutionary ecology perspective. Advances in Insect Physiology, 32, 1–48.
Speakman, J. R. (2005). Body size, energy metabolism and lifespan. Journal of Experimental Biology, 208, 1717–1730.
Via, S., & Lande, R. (1985). Genotype–environment interaction and the evolution of phenotypic plasticity. Evolution, 39(3), 505–522.
Ye, Y. H., Chenoweth, S. F., & McGraw, E. A. (2009). Effective but costly, evolved mechanisms of defense against a virulent opportunistic pathogen in Drosophila melanogaster. PloS Pathogens, 5(4), e1000385.
Zerofsky, M., Harel, E., Silverman, N., & Tatar, M. (2005). Aging of the innate immune response in Drosophila melanogaster. Aging Cell, 4(2), 103–108.
Acknowledgements
This study was funded by the Academy of Finland to M.J.R., the Finnish Cultural Foundation’s Varsinais-Suomi Regional Fund to T.M.V., Competitive Research Funding of the Pirkanmaa Hospital District to A.K. and by grants from the Academy of Finland, the Foundation for Pediatric Research, Sigrid Juselius Foundation, Emil Aaltonen Foundation and Competitive Research Funding of the Pirkanmaa Hospital District to M.R.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Valtonen, T.M., Kleino, A., Rämet, M. et al. Starvation Reveals Maintenance Cost of Humoral Immunity. Evol Biol 37, 49–57 (2010). https://doi.org/10.1007/s11692-009-9078-3
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11692-009-9078-3