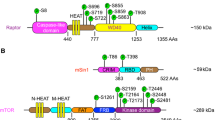
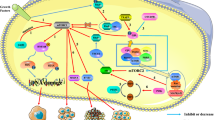
Abstract
The mammalian target of rapamycin (mTOR) critically regulates several essential biological functions, such as cell growth, metabolism, survival, and immune response by forming two important complexes, namely, mTOR complex 1 (mTORC1) and complex 2 (mTORC2). mTOR signaling is often dysregulated in cancers and has been considered an attractive cancer therapeutic target. Great efforts have been made to develop efficacious mTOR inhibitors, particularly mTOR kinase inhibitors, which suppress mTORC1 and mTORC2; however, major success has not been achieved. With the strong scientific rationale, the intriguing question is why cancers are insensitive or not responsive to mTOR-targeted cancer therapy in clinics. Beyond early findings on induced activation of PI3K/Akt, MEK/ERK, and Mnk/eIF4E survival signaling pathways that compromise the efficacy of rapalog-based cancer therapy, recent findings on the essential role of GSK3 in mediating cancer cell response to mTOR inhibitors and mTORC1 inhibition-induced upregulation of PD-L1 in cancer cells may provide some explanations. These new findings may also offer us the opportunity to rationally utilize mTOR inhibitors in cancer therapy. Further elucidation of the biology of complicated mTOR networks may bring us the hope to develop effective therapeutic strategies with mTOR inhibitors against cancer.
Article PDF
Similar content being viewed by others
Explore related subjects
Discover the latest articles and news from researchers in related subjects, suggested using machine learning.Avoid common mistakes on your manuscript.
References
Guertin DA, Sabatini DM. Defining the role of mTOR in cancer. Cancer Cell 2007; 12(1): 9–22
Oh WJ, Jacinto E. mTOR complex 2 signaling and functions. Cell Cycle 2011; 10(14): 2305–2316
Laplante M, Sabatini DM. mTOR signaling at a glance. J Cell Sci 2009; 122(Pt 20): 3589–3594
Cai W, Ye Q, She QB. Loss of 4E-BP1 function induces EMT and promotes cancer cell migration and invasion via cap-dependent translational activation of snail. Oncotarget 2014; 5(15): 6015–6027
Guertin DA, Stevens DM, Saitoh M, Kinkel S, Crosby K, Sheen JH, Mullholland DJ, Magnuson MA, Wu H, Sabatini DM. mTOR complex 2 is required for the development of prostate cancer induced by Pten loss in mice. Cancer Cell 2009; 15(2): 148–159
Lee K, Nam KT, Cho SH, Gudapati P, Hwang Y, Park DS, Potter R, Chen J, Volanakis E, Boothby M. Vital roles of mTOR complex 2 in Notch-driven thymocyte differentiation and leukemia. J Exp Med 2012; 209(4): 713–728
Roulin D, Cerantola Y, Dormond-Meuwly A, Demartines N, Dormond O. Targeting mTORC2 inhibits colon cancer cell proliferation in vitro and tumor formation in vivo. Mol Cancer 2010; 9(1): 57
Sun SY. Impact of genetic alterations on mTOR-targeted cancer therapy. Chin J Cancer 2013; 32(5): 270–274
Wang X, Sun SY. Enhancing mTOR-targeted cancer therapy. Expert Opin Ther Targets 2009; 13(10): 1193–1203
Abraham RT, Gibbons JJ. The mammalian target of rapamycin signaling pathway: twists and turns in the road to cancer therapy. Clin Cancer Res 2007; 13(11): 3109–3114
Sun SY. mTOR kinase inhibitors as potential cancer therapeutic drugs. Cancer Lett 2013; 340(1): 1–8
Zhang YJ, Duan Y, Zheng XF. Targeting the mTOR kinase domain: the second generation of mTOR inhibitors. Drug Discov Today 2011; 16(7–8): 325–331
Rodrik-Outmezguine VS, Okaniwa M, Yao Z, Novotny CJ, McWhirter C, Banaji A, Won H, Wong W, Berger M, de Stanchina E, Barratt DG, Cosulich S, Klinowska T, Rosen N, Shokat KM. Overcoming mTOR resistance mutations with a new-generation mTOR inhibitor. Nature 2016; 534(7606): 272–276
Sun SY, Rosenberg LM, Wang X, Zhou Z, Yue P, Fu H, Khuri FR. Activation of Akt and eIF4E survival pathways by rapamycin-mediated mammalian target of rapamycin inhibition. Cancer Res 2005; 65(16): 7052–7058
O’Reilly KE, Rojo F, She QB, Solit D, Mills GB, Smith D, Lane H, Hofmann F, Hicklin DJ, Ludwig DL, Baselga J, Rosen N. mTOR inhibition induces upstream receptor tyrosine kinase signaling and activates Akt. Cancer Res 2006; 66(3): 1500–1508
Shi Y, Yan H, Frost P, Gera J, Lichtenstein A. Mammalian target of rapamycin inhibitors activate the AKT kinase in multiple myeloma cells by up-regulating the insulin-like growth factor receptor/insulin receptor substrate-1/phosphatidylinositol 3-kinase cascade. Mol Cancer Ther 2005; 4(10): 1533–1540
Wan X, Harkavy B, Shen N, Grohar P, Helman LJ. Rapamycin induces feedback activation of Akt signaling through an IGF-1R-dependent mechanism. Oncogene 2007; 26(13): 1932–1940
Wang X, Yue P, Kim YA, Fu H, Khuri FR, Sun SY. Enhancing mammalian target of rapamycin (mTOR)-targeted cancer therapy by preventing mTOR/raptor inhibition-initiated, mTOR/rictor-independent Akt activation. Cancer Res 2008; 68(18): 7409–7418
Duran I, Kortmansky J, Singh D, Hirte H, Kocha W, Goss G, Le L, Oza A, Nicklee T, Ho J, Birle D, Pond GR, Arboine D, Dancey J, Aviel-Ronen S, Tsao MS, Hedley D, Siu LL. A phase II clinical and pharmacodynamic study of temsirolimus in advanced neuroendocrine carcinomas. Br J Cancer 2006; 95(9): 1148–1154
Tabernero J, Rojo F, Calvo E, Burris H, Judson I, Hazell K, Martinelli E, Ramony Cajal S, Jones S, Vidal L, Shand N, Macarulla T, Ramos FJ, Dimitrijevic S, Zoellner U, Tang P, Stumm M, Lane HA, Lebwohl D, Baselga J. Dose- and schedule-dependent inhibition of the mammalian target of rapamycin pathway with everolimus: a phase I tumor pharmacodynamic study in patients with advanced solid tumors. J Clin Oncol 2008; 26(10): 1603–1610
Easton JB, Kurmasheva RT, Houghton PJ. IRS-1: auditing the effectiveness of mTOR inhibitors. Cancer Cell 2006; 9(3): 153–155
Wang X, Yue P, Tao H, Sun SY. Inhibition of p70S6K does not mimic the enhancement of Akt phosphorylation by rapamycin. Heliyon 2017; 3(8): e00378
Li Y, Wang X, Yue P, Tao H, Ramalingam SS, Owonikoko TK, Deng X, Wang Y, Fu H, Khuri FR, Sun SY. Protein phosphatase 2A and DNA-dependent protein kinase are involved in mediating rapamycin-induced Akt phosphorylation. J Biol Chem 2013; 288 (19): 13215–13224
Carracedo A, Ma L, Teruya-Feldstein J, Rojo F, Salmena L, Alimonti A, Egia A, Sasaki AT, Thomas G, Kozma SC, Papa A, Nardella C, Cantley LC, Baselga J, Pandolfi PP. Inhibition of mTORC1 leads to MAPK pathway activation through a PI3K-dependent feedback loop in human cancer. J Clin Invest 2008; 118 (9): 3065–3074
Wang X, Hawk N, Yue P, Kauh J, Ramalingam SS, Fu H, Khuri FR, Sun SY. Overcoming mTOR inhibition-induced paradoxical activation of survival signaling pathways enhances mTOR inhibitors’ anticancer efficacy. Cancer Biol Ther 2008; 7(12): 1952–1958
Wang X, Yue P, Chan CB, Ye K, Ueda T, Watanabe-Fukunaga R, Fukunaga R, Fu H, Khuri FR, Sun SY. Inhibition of mammalian target of rapamycin induces phosphatidylinositol 3 kinase-dependent and Mnk-mediated eIF4E phosphorylation. Mol Cell Biol 2007; 27(21): 7405–7413
Frame S, Cohen P. GSK3 takes centre stage more than 20 years after its discovery. Biochem J 2001; 359(Pt 1): 1–16
Mills CN, Nowsheen S, Bonner JA, Yang ES. Emerging roles of glycogen synthase kinase 3 in the treatment of brain tumors. Front Mol Neurosci 2011; 4: 47
Mishra R. Glycogen synthase kinase 3ß: can it be a target for oral cancer. Mol Cancer 2010; 9(1): 144
Cohen P, Frame S. The renaissance of GSK3. Nat Rev Mol Cell Biol 2001; 2(10): 769–776
McCubrey JA, Davis NM, Abrams SL, Montalto G, Cervello M, Basecke J, Libra M, Nicoletti F, Cocco L, Martelli AM, Steelman LS. Diverse roles of GSK-3: tumor promoter-tumor suppressor, target in cancer therapy. Adv Biol Regul 2014; 54: 176–196
Medina M, Wandosell F. Deconstructing GSK-3: the fine regulation of its activity. Int J Alzheimers Dis 2011; 2011: 479249
Shin S, Wolgamott L, Yu Y, Blenis J, Yoon SO. Glycogen synthase kinase (GSK)-3 promotes p70 ribosomal protein S6 kinase (p70S6K) activity and cell proliferation. Proc Natl Acad Sci USA 2011; 108(47): E1204–E1213
Koo J, Wang X, Owonikoko TK, Ramalingam SS, Khuri FR, Sun SY. GSK3 is required for rapalogs to induce degradation of some oncogenic proteins and to suppress cancer cell growth. Oncotarget 2015; 6(11): 8974–8987
Koo J, Yue P, Gal AA, Khuri FR, Sun SY. Maintaining glycogen synthase kinase-3 activity is critical for mTOR kinase inhibitors to inhibit cancer cell growth. Cancer Res 2014; 74(9): 2555–2568
Zhang S, Qian G, Zhang QQ, Yao Y, Wang D, Chen ZG, Wang LJ, Chen M, Sun SY. mTORC2 suppresses GSK3-dependent Snail degradation to positively regulate cancer cell invasion and metastasis. Cancer Res 2019; 79(14): 3725–3736
Inoki K, Ouyang H, Zhu T, Lindvall C, Wang Y, Zhang X, Yang Q, Bennett C, Harada Y, Stankunas K, Wang CY, He X, MacDougald OA, You M, Williams BO, Guan KL. TSC2 integrates Wnt and energy signals via a coordinated phosphorylation by AMPK and GSK3 to regulate cell growth. Cell 2006; 126(5): 955–968
Zhang HH, Lipovsky AI, Dibble CC, Sahin M, Manning BD. S6K1 regulates GSK3 under conditions of mTOR-dependent feedback inhibition of Akt. Mol Cell 2006; 24(2): 185–197
Koo J, Yue P, Deng X, Khuri FR, Sun SY. mTOR complex 2 stabilizes Mcl-1 protein by suppressing its GSK3-dependent and SCF-FBXW7-mediated degradation. Mol Cell Biol 2015; 35: 2344–2355
Li S, Oh YT, Yue P, Khuri FR, Sun SY. Inhibition of mTOR complex 2 induces GSK3/FBXW7-dependent degradation of sterol regulatory element-binding protein 1 (SREBP1) and suppresses lipogenesis in cancer cells. Oncogene 2016; 35(5): 642–650
Welcker M, Clurman BE. FBW7 ubiquitin ligase: a tumour suppressor at the crossroads of cell division, growth and differentiation. Nat Rev Cancer 2008; 8(2): 83–93
Inuzuka H, Shaik S, Onoyama I, Gao D, Tseng A, Maser RS, Zhai B, Wan L, Gutierrez A, Lau AW, Xiao Y, Christie AL, Aster J, Settleman J, Gygi SP, Kung AL, Look T, Nakayama KI, DePinho RA, Wei W. SCF(FBW7) regulates cellular apoptosis by targeting MCL1 for ubiquitylation and destruction. Nature 2011; 471(7336): 104–109
Takahashi-Yanaga F, Sasaguri T. GSK-3ß regulates cyclin D1 expression: a new target for chemotherapy. Cell Signal 2008; 20(4): 581–589
Cuadrado A. Structural and functional characterization of Nrf2 degradation by glycogen synthase kinase 3/β-TrCP. Free Radic Biol Med 2015; 88(Pt B): 147–157
Xu C, Kim NG, Gumbiner BM. Regulation of protein stability by GSK3 mediated phosphorylation. Cell Cycle 2009; 8(24): 4032–4039
Zhou BP, Deng J, Xia W, Xu J, Li YM, Gunduz M, Hung MC. Dual regulation of Snail by GSK-3β-mediated phosphorylation in control of epithelial-mesenchymal transition. Nat Cell Biol 2004; 6(10): 931–940
Sarbassov DD, Ali SM, Sengupta S, Sheen JH, Hsu PP, Bagley AF, Markhard AL, Sabatini DM. Prolonged rapamycin treatment inhibits mTORC2 assembly and Akt/PKB. Mol Cell 2006; 22(2): 159–168
Barilli A, Visigalli R, Sala R, Gazzola GC, Parolari A, Tremoli E, Bonomini S, Simon A, Closs EI, Dall’Asta V, Bussolati O. In human endothelial cells rapamycin causes mTORC2 inhibition and impairs cell viability and function. Cardiovasc Res 2008; 78(3): 563–571
Rosner M, Hengstschläger M. Cytoplasmic and nuclear distribution of the protein complexes mTORC1 and mTORC2: rapamycin triggers dephosphorylation and delocalization of the mTORC2 components rictor and sin1. Hum Mol Genet 2008; 17(19): 2934–2948
Barquilla A, Crespo JL, Navarro M. Rapamycin inhibits trypanosome cell growth by preventing TOR complex 2 formation. Proc Natl Acad Sci USA 2008; 105(38): 14579–14584
Barlow AD, Xie J, Moore CE, Campbell SC, Shaw JA, Nicholson ML, Herbert TP. Rapamycin toxicity in MIN6 cells and rat and human islets is mediated by the inhibition of mTOR complex 2 (mTORC2). Diabetologia 2012; 55(5): 1355–1365
Lamming DW, Ye L, Katajisto P, Goncalves MD, Saitoh M, Stevens DM, Davis JG, Salmon AB, Richardson A, Ahima RS, Guertin DA, Sabatini DM, Baur JA. Rapamycin-induced insulin resistance is mediated by mTORC2 loss and uncoupled from longevity. Science 2012; 335(6076): 1638–1643
Hong SM, Park CW, Cha HJ, Kwon JH, Yun YS, Lee NG, Kim DG, Nam HG, Choi KY. Rapamycin inhibits both motility through down-regulation of p-STAT3 (S727) by disrupting the mTORC2 assembly and peritoneal dissemination in sarcomatoid cholangio-carcinoma. Clin Exp Metastasis 2013; 30(2): 177–187
Ding Q, He X, Xia W, Hsu JM, Chen CT, Li LY, Lee DF, Yang JY, Xie X, Liu JC, Hung MC. Myeloid cell leukemia-1 inversely correlates with glycogen synthase kinase-3β activity and associates with poor prognosis in human breast cancer. Cancer Res 2007; 67 (10): 4564–4571
Chung R, Peters AC, Armanious H, Anand M, Gelebart P, Lai R. Biological and clinical significance of GSK-3β in mantle cell lymphoma—an immunohistochemical study. Int J Clin Exp Pathol 2010; 3(3): 244–253 PMID:20224723
Cho YJ, Kim JH, Yoon J, Cho SJ, Ko YS, Park JW, Lee HS, Lee HE, Kim WH, Lee BL. Constitutive activation of glycogen synthase kinase-3β correlates with better prognosis and cyclin-dependent kinase inhibitors in human gastric cancer. BMC Gastroenterol 2010; 10(1): 91
Qiao G, Le Y, Li J, Wang L, Shen F. Glycogen synthase kinase-3β is associated with the prognosis of hepatocellular carcinoma and may mediate the influence of type 2 diabetes mellitus on hepatocellular carcinoma. PLoS One 2014; 9(8): e105624
Yeh CH, Bellon M, Nicot C. FBXW7: a critical tumor suppressor of human cancers. Mol Cancer 2018; 17(1): 115
Davis RJ, Welcker M, Clurman BE. Tumor suppression by the Fbw7 ubiquitin ligase: mechanisms and opportunities. Cancer Cell 2014; 26(4): 455–464
Guan J, Lim KS, Mekhail T, Chang CC. Programmed death ligand-1 (PD-L1) expression in the programmed death receptor-1 (PD-1)/PD-L1 blockade: a key player against various cancers. Arch Pathol Lab Med 2017; 141(6): 851–861
Benson DM Jr, Bakan CE, Mishra A, Hofmeister CC, Efebera Y, Becknell B, Baiocchi RA, Zhang J, Yu J, Smith MK, Greenfield CN, Porcu P, Devine SM, Rotem-Yehudar R, Lozanski G, Byrd JC, Caligiuri MA. The PD-1/PD-L1 axis modulates the natural killer cell versus multiple myeloma effect: a therapeutic target for CT-011, a novel monoclonal anti-PD-1 antibody. Blood 2010; 116(13): 2286–2294
Gordon SR, Maute RL, Dulken BW, Hutter G, George BM, McCracken MN, Gupta R, Tsai JM, Sinha R, Corey D, Ring AM, Connolly AJ, Weissman IL. PD-1 expression by tumour-associated macrophages inhibits phagocytosis and tumour immunity. Nature 2017; 545(7655): 495–499
Iwai Y, Hamanishi J, Chamoto K, Honjo T. Cancer immunotherapies targeting the PD-1 signaling pathway. J Biomed Sci 2017; 24 (1): 26
Somasundaram A, Burns TF. The next generation of immunotherapy: keeping lung cancer in check. J Hematol Oncol 2017; 10(1): 87
Stambrook PJ, Maher J, Farzaneh F. Cancer immunotherapy: whence and whither. Mol Cancer Res 2017; 15(6): 635–650
Lastwika KJ, Wilson W 3rd, Li QK, Norris J, Xu H, Ghazarian SR, Kitagawa H, Kawabata S, Taube JM, Yao S, Liu LN, Gills JJ, Dennis PA. Control of PD-L1 expression by oncogenic activation of the AKT-mTOR pathway in non-small cell lung cancer. Cancer Res 2016; 76(2): 227–238
Deng L, Qian G, Zhang S, Zheng H, Fan S, Lesinski GB, Owonikoko TK, Ramalingam SS, Sun SY. Inhibition of mTOR complex 1/p70 S6 kinase signaling elevates PD-L1 levels in human cancer cells through enhancing protein stabilization accompanied with enhanced β-TrCP degradation. Oncogene 2019; 38(35): 6270–6282
Hirayama Y, Gi M, Yamano S, Tachibana H, Okuno T, Tamada S, Nakatani T, Wanibuchi H. Anti-PD-L1 treatment enhances antitumor effect of everolimus in a mouse model of renal cell carcinoma. Cancer Sci 2016; 107(12): 1736–1744
Zhang C, Duan Y, Xia M, Dong Y, Chen Y, Zheng L, Chai S, Zhang Q, Wei Z, Liu N, Wang J, Sun C, Tang Z, Cheng X, Wu J, Wang G, Zheng F, Laurence A, Li B, Yang XP. TFEB mediates immune evasion and resistance to mTOR inhibition of renal cell carcinoma via induction of PD-L1. Clin Cancer Res 2019; 25(22): 6827–6838
Hua H, Kong Q, Zhang H, Wang J, Luo T, Jiang Y. Targeting mTOR for cancer therapy. J Hematol Oncol 2019; 12(1): 71
Holder AM, Akcakanat A, Adkins F, Evans K, Chen H, Wei C, Milton DR, Li Y, Do KA, Janku F, Meric-Bernstam F. Epithelial to mesenchymal transition is associated with rapamycin resistance. Oncotarget 2015; 6(23): 19500–19513
Venkatesan S, Hoogstraat M, Caljouw E, Pierson T, Spoor JK, Zeneyedpour L, Dubbink HJ, Dekker LJ, van der Kaaij M, Kloezeman J, Berghauser Pont LM, Besselink NJ, Luider TM, Joore J, Martens JW, Lamfers ML, Sleijfer S, Leenstra S. TP53 mutated glioblastoma stem-like cell cultures are sensitive to dual mTORC1/2 inhibition while resistance in TP53 wild type cultures can be overcome by combined inhibition of mTORC1/2 and Bcl-2. Oncotarget 2016; 7(36): 58435–58444
Tan J, Li Z, Lee PL, Guan P, Aau MY, Lee ST, Feng M, Lim CZ, Lee EY, Wee ZN, Lim YC, Karuturi RK, Yu Q. PDK1 signaling toward PLK1-MYC activation confers oncogenic transformation, tumor-initiating cell activation, and resistance to mTOR-targeted therapy. CancerDiscov 2013; 3(10): 1156–1171
Liu Y, Zhang X, Liu P, Zhang J. Drug sensitivity research ofmTOR inhibitor on breast cancer stem cells. Natl Med J China (Zhonghua Yi Xue Za Zhi) 2015; 95(24): 1910–1914 (in Chinese)
Lin F, de Gooijer MC, Hanekamp D, Chandrasekaran G, Buil LC, Thota N, Sparidans RW, Beijnen JH, Würdinger T, van Tellingen O. PI3K-mTOR pathway inhibition exhibits efficacy against highgrade glioma in clinically relevant mouse models. Clin Cancer Res 2017; 23(5): 1286–1298
Wang J, Yang DH, Yang Y, Wang JQ, Cai CY, Lei ZN, Teng QX, Wu ZX, Zhao L, Chen ZS. Overexpression of ABCB1 transporter confers resistance to mTOR inhibitor WYE-354 in cancer cells. Int J Mol Sci 2020; 21(4): E1387
Lin F, Buil L, Sherris D, Beijnen JH, van Tellingen O. Dual mTORC1 and mTORC2 inhibitor Palomid 529 penetrates the blood-brain barrier without restriction by ABCB1 and ABCG2. Int J Cancer 2013; 133(5): 1222–1233
Lauretti E, Dincer O, Pratico D. Glycogen synthase kinase-3 signaling in Alzheimer’s disease. Biochim Biophys Acta Mol Cell Res 2020; 1867(5): 118664
Duda P, Wiśniewski J, Wójtowicz T, Wójcicka O, Jaśkiewicz M, Drulis-Fajdasz D, Rakus D, McCubrey JA, Gizak A. Targeting GSK3 signaling as a potential therapy ofneurodegenerative diseases and aging. Expert Opin Ther Targets 2018; 22(10): 833–848
Lynch JT, Polanska UM, Hancox U, Delpuech O, Maynard J, Trigwell C, Eberlein C, Lenaghan C, Polanski R, Avivar-Valderas A, Cumberbatch M, Klinowska T, Critchlow SE, Cruzalegui F, Barry ST. Combined inhibition of PI3Kβ and mTOR inhibits growth of PTEN-null tumors. Mol Cancer Ther 2018; 17(11): 2309–2319
Kim H, Lee SJ, Lee IK, Min SC, Sung HH, Jeong BC, Lee J, Park SH. Synergistic effects of combination therapy with AKT and mTOR inhibitors on bladder cancer cells. Int J Mol Sci 2020; 21(8): E2825
Mazzoletti M, Bortolin F, Brunelli L, Pastorelli R, Di Giandomenico S, Erba E, Ubezio P, Broggini M. Combination of PI3K/mTOR inhibitors: antitumor activity and molecular correlates. Cancer Res 2011; 71(13): 4573–4584
Mise J, Dembitz V, Banfic H, Visnjic D. Combined inhibition of PI3K and mTOR exerts synergistic antiproliferative effect, but diminishes differentiative properties of rapamycin in acute myeloid leukemia cells. Pathol Oncol Res 2011; 17(3): 645–656
Arnold A, Yuan M, Price A, Harris L, Eberhart CG, Raabe EH. Synergistic activity of mTORC1/2 kinase and MEK inhibitors suppresses pediatric low-grade glioma tumorigenicity and vascularity. Neuro-oncol 2020; 22(4): 563–574
Liu X, Hu J, Song X, Utpatel K, Zhang Y, Wang P, Lu X, Zhang J, Xu M, Su T, Che L, Wang J, Evert M, Calvisi DF, Chen X. Combined treatment with MEK and mTOR inhibitors is effective in in vitro and in vivo models of hepatocellular carcinoma. Cancers (Basel) 2019; 11(7): E930
Chadwick ML, Lane A, Thomas D, Smith AR, White AR, Davidson D, Feng Y, Boscolo E, Zheng Y, Adams DM, Gupta A, Veillette A, Chow LML. Combined mTOR and MEK inhibition is an effective therapy in a novel mouse model for angiosarcoma. Oncotarget 2018; 9(37): 24750–24765
Andersen NJ, Boguslawski EB, Kuk CY, Chambers CM, Duesbery NS. Combined inhibition of MEK and mTOR has a synergic effect on angiosarcoma tumorgrafts. Int J Oncol 2015; 47(1): 71–80
Araki K, Youngblood B, Ahmed R. The role of mTOR in memory CD8 T-cell differentiation. Immunol Rev 2010; 235(1): 234–243
Chi H. Regulation and function of mTOR signalling in T cell fate decisions. Nat Rev Immunol 2012; 12(5): 325–338
Chapman NM, Chi H. mTOR signaling, Tregs and immune modulation. Immunotherapy 2014; 6(12): 1295–1311
Fantus D, Thomson AW. Evolving perspectives of mTOR complexes in immunity and transplantation. Am J Transplant 2015; 15(4): 891–902
Araki K, Turner AP, Shaffer VO, Gangappa S, Keller SA, Bachmann MF, Larsen CP, Ahmed R. mTOR regulates memory CD8 T-cell differentiation. Nature 2009; 460(7251): 108–112
Mannick JB, Del Giudice G, Lattanzi M, Valiante NM, Praestgaard J, Huang B, Lonetto MA, Maecker HT, Kovarik J, Carson S, Glass DJ, Klickstein LB. mTOR inhibition improves immune function in the elderly. Sci Transl Med 2014; 6(268): 268ra179
Beziaud L, Mansi L, Ravel P, Marie-Joseph EL, Laheurte C, Rangan L, Bonnefoy F, Pallandre JR, Boullerot L, Gamonet C, Vrecko S, Queiroz L, Maurina T, Mouillet G, Hon TN, Curtit E, Royer B, Gaugler B, Bayry J, Tartour E, Thiery-Vuillemin A, Pivot X, Borg C, Godet Y, Adotévi O. Rapalogs efficacy relies on the modulation of antitumor T-cell immunity. Cancer Res 2016; 76(14): 4100–4112
Amiel E, Everts B, Freitas TC, King IL, Curtis JD, Pearce EL, Pearce EJ. Inhibition of mechanistic target of rapamycin promotes dendritic cell activation and enhances therapeutic autologous vaccination in mice. J Immunol 2012; 189(5): 2151–2158
Thomas DL, Doty R, Tosic V, Liu J, Kranz DM, McFadden G, Macneill AL, Roy EJ. Myxoma virus combined with rapamycin treatment enhances adoptive T cell therapy for murine melanoma brain tumors. Cancer Immunol Immunother 2011; 60(10): 1461–1472
Diken M, Kreiter S, Vascotto F, Selmi A, Attig S, Diekmann J, Huber C, Türeci Ö, Sahin U. mTOR inhibition improves antitumor effects of vaccination with antigen-encoding RNA. Cancer Immunol Res 2013; 1(6): 386–392
Mineharu Y, Kamran N, Lowenstein PR, Castro MG. Blockade of mTOR signaling via rapamycin combined with immunotherapy augments antiglioma cytotoxic and memory T-cell functions. Mol Cancer Ther 2014; 13(12): 3024–3036
Moore EC, Cash HA, Caruso AM, Uppaluri R, Hodge JW, Van Waes C, Allen CT. Enhanced tumor control with combination mTOR and PD-L1 inhibition in syngeneic oral cavity cancers. Cancer Immunol Res 2016; 4(7): 611–620
Acknowledgements
I am grateful to Dr. Karin A. Albuerne in my laboratory for editing the manuscript. Shi-Yong Sun is a Georgia Research Alliance Distinguished Cancer Scientist.
Author information
Authors and Affiliations
Corresponding author
Additional information
Compliance with ethics guidelines
Shi-Yong Sun declares no conflict of interest. This manuscript is a review article and does not involve a research protocol requiring approval by the relevant institutional review board or ethics committee.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made.
The images or other third party material in this article are included in the article’s Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder.
To view a copy of this licence, visit https://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Sun, SY. mTOR-targeted cancer therapy: great target but disappointing clinical outcomes, why?. Front. Med. 15, 221–231 (2021). https://doi.org/10.1007/s11684-020-0812-7
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11684-020-0812-7