Abstract
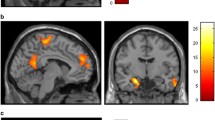
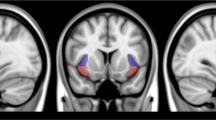
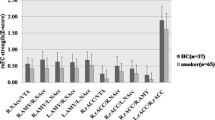
Cigarette smoking is associated with elevated risk of disease and mortality and contributes to heavy healthcare-related economic burdens. The nucleus accumbens is implicated in numerous reward-related behaviors, including reinforcement learning and incentive salience. The established functional connectivity of the accumbens includes regions associated with motivation, valuation, and affective processing. Although the high comorbidity of cigarette smoking with drinking behaviors may collectively affect brain activity, there could be independent effects of smoking in alcohol use disorder that impact brain function and behavior. We hypothesized that smoking status, independent of alcohol use, would be associated with aberrations of nucleus accumbens functional connectivity to brain regions that facilitate reward processing, salience attribution, and inhibitory control. Resting state functional magnetic resonance imaging data from thirty-one nonsmokers and nineteen smoking individuals were analyzed using seed-based correlations of the bilateral accumbens with all other brain voxels. Statistical models accounted for drinks consumed per week. The smoking group demonstrated significantly higher functional connectivity between the left accumbens and the bilateral insula and anterior cingulate cortex, as well as hyperconnectivity between the right accumbens and the insula. Confirmatory analyses using the insula and cingulate clusters generated from the original analysis as seed regions reproduced the hyperconnectivity in smokers between the bilateral insular regions and the accumbens. In conclusion, smoking status had distinct effects on neural activity; hyperconnectivity between the accumbens and insula in smokers may reflect enhanced encoding of the reinforcing effects of smoking and greater orientation toward smoking-associated stimuli.

Similar content being viewed by others
Data availability
No datasets were generated or analysed during the current study.
References
Adams, S. (2017). Psychopharmacology of tobacco and alcohol comorbidity: A review of current evidence. Current Addiction Reports, 4, 25–34.
Albrecht, D. S., Kareken, D. A., & Yoder, K. K. (2013). Effects of smoking on D₂/D₃ striatal receptor availability in alcoholics and social drinkers. Brain Imaging Behav, 7(3), 326–334. https://doi.org/10.1007/s11682-013-9233-4.
Avants, B. B., Tustison, N. J., Song, G., & Gee, J. C. (2009). Ants: Open-source tools for normalization and neuroanatomy. HeanetIe, 10, 1–11.
Babor, T. F., & Robaina, K. (2016). The Alcohol Use Disorders Identification Test (AUDIT): A review of graded severity algorithms and national adaptations.
Baliki, M. N., Mansour, A., Baria, A. T., Huang, L., Berger, S. E., Fields, H. L., & Apkarian, A. V. (2013). Parceling human accumbens into putative core and shell dissociates encoding of values for reward and pain. Journal of Neuroscience, 33(41), 16383–16393. https://doi.org/10.1523/JNEUROSCI.1731-13.2013.
Bauer, U. E., Briss, P. A., Goodman, R. A., & Bowman, B. A. (2014). Prevention of chronic disease in the 21st century: Elimination of the leading preventable causes of premature death and disability in the USA. The Lancet, 384(9937), 45–52. https://doi.org/10.1016/S0140-6736(14)60648-6.
Berridge, K. C., & Robinson, T. E. (2016). Liking, wanting, and the incentive-sensitization theory of addiction. American Psychologist, 71(8), 670–679. https://doi.org/10.1037/amp0000059.
Billieux, J., Gay, P., Rochat, L., Khazaal, Y., Zullino, D., & Van der Linden, M. (2010). Lack of inhibitory control predicts cigarette smoking dependence: Evidence from a non-deprived sample of light to moderate smokers. Drug and Alcohol Dependence, 112(1–2), 164–167.
Bucholz, K. K., Cadoret, R., Cloninger, C. R., Dinwiddie, S. H., Hesselbrock, V., Nurnberger Jr, J., Reich, T., Schmidt, I., & Schuckit, M. A. (1994). A new, semi-structured psychiatric interview for use in genetic linkage studies: A report on the reliability of the SSAGA. Journal of Studies on Alcohol, 55(2), 149–158.
Burling, T. A., & Ziff, D. C. (1988). Tobacco smoking: A comparison between alcohol and drug abuse inpatients. Addictive Behaviors, 13(2), 185–190. https://doi.org/0306-4603(88)90010-X [pii]
Cartmell, S. C., Tian, Q., Thio, B. J., Leuze, C., Ye, L., Williams, N. R., Yang, G., Ben-Dor, G., Deisseroth, K., Grill, W. M., McNab, J. A., & Halpern, C. H. (2019). Multimodal characterization of the human nucleus accumbens. Neuroimage, 198, 137–149. https://doi.org/10.1016/j.neuroimage.2019.05.019.
Cohen, M. X., Schoene-Bake, J. C., Elger, C. E., & Weber, B. (2009). Connectivity-based segregation of the human striatum predicts personality characteristics. Nature Neuroscience, 12(1), 32–34.
Cosgrove, K. P., Wang, S., Kim, S. J., McGovern, E., Nabulsi, N., Gao, H., Labaree, D., Tagare, H. D., Sullivan, J. M., & Morris, E. D. (2014). Sex differences in the brain’s dopamine signature of cigarette smoking. Journal of Neuroscience, 34(50), 16851–16855. https://doi.org/10.1523/JNEUROSCI.3661-14.2014.
Cyders, M. A., Littlefield, A. K., Coffey, S., & Karyadi, K. A. (2014). Examination of a short English version of the UPPS-P Impulsive Behavior Scale. Addictive Behaviors, 39(9), 1372–1376.
D’Souza, M. S., & Markou, A. (2011). Neuronal mechanisms underlying development of nicotine dependence: Implications for novel smoking-cessation treatments. Addiction Science & Clinical Practice, 6(1), 4.
Durazzo, T. C., & Meyerhoff, D. J. (2017). Psychiatric, demographic, and brain morphological predictors of relapse after treatment for an alcohol use disorder. Alcoholism: Clinical and Experimental Research, 41(1), 107–116.
Durazzo, T. C., Abadjian, L., Kincaid, A., Bilovsky-Muniz, T., Boreta, L., & Gauger, G. E. (2013). The influence of chronic cigarette smoking on neurocognitive recovery after mild traumatic brain injury. Journal of Neurotrauma, 30(11), 1013–1022. https://doi.org/10.1089/neu.2012.2676.
Durazzo, T. C., Pennington, D. L., Schmidt, T. P., & Meyerhoff, D. J. (2014). Effects of cigarette smoking history on neurocognitive recovery over 8 months of abstinence in alcohol-dependent individuals. Alcoholism, Clinical and Experimental Research, 38(11), 2816–2825. https://doi.org/10.1111/acer.12552.
Durazzo, T. C., Meyerhoff, D. J., Yoder, K. K., & Murray, D. E. (2017). Cigarette smoking is associated with amplified age-related volume loss in subcortical brain regions. Drug and Alcohol Dependence, 177, 228–236.
Durazzo, T. C., Meyerhoff, D. J., & Yoder, K. K. (2018). Cigarette smoking is associated with cortical thinning in anterior frontal regions, insula and regions showing atrophy in early Alzheimer’s Disease. Drug and Alcohol Dependence, 192, 277–284. https://doi.org/10.1016/j.drugalcdep.2018.08.009.
Eklund, A., Nichols, T. E., & Knutsson, H. (2016). Cluster failure: Why fMRI inferences for spatial extent have inflated false-positive rates. Proceedings of the National Academy of Sciences, 113(28), 7900–7905.
Etter, J. F. (2005). A self-administered questionnaire to measure cigarette withdrawal symptoms: The cigarette Withdrawal Scale. Nicotine & Tobacco Research, 7(1), 47–57.
Falk, D. E., Yi, H. Y., & Hiller-Sturmhofel, S. (2006). An epidemiologic analysis of co-occurring alcohol and tobacco use and disorders: Findings from the National Epidemiologic Survey on Alcohol and related conditions. Alcohol Research & Health, 29(3), 162–171. http://www.ncbi.nlm.nih.gov/pubmed/17373404.
Gerchen, M. F., Weiss, F., Kirsch, M., Rentsch, A., Halli, P., Kiefer, F., & Kirsch, P. (2021). Dynamic frontostriatal functional peak connectivity (in alcohol use disorder). Human Brain Mapping, 42(1), 36–46. https://doi.org/10.1002/hbm.25201.
Ghahremani, D. G., Pochon, J. B., Diaz, P., Tyndale, M., Dean, R. F., A. C., & London, E. D. (2021). Functional connectivity of the anterior insula during withdrawal from cigarette smoking. Neuropsychopharmacology: Official Publication of the American College of Neuropsychopharmacology, 46(12), 2083–2089. https://doi.org/10.1038/s41386-021-01036-z.
Ghahremani, D. G., Pochon, J. F., Diaz, M. P., Tyndale, R. F., Dean, A. C., & London, E. D. (2023). Nicotine dependence and insula subregions: Functional connectivity and cue-induced activation. Neuropsychopharmacology: Official Publication of the American College of Neuropsychopharmacology, 48(6), 936–945. https://doi.org/10.1038/s41386-023-01528-0.
Gordon, E. M., Laumann, T. O., Marek, S., Newbold, D. J., Hampton, J. M., Seider, N. A., Montez, D. F., Nielsen, A. M., Van, A. N., Zheng, A., Miller, R., Siegel, J. S., Kay, B. P., Snyder, A. Z., Greene, D. J., Schlaggar, B. L., Petersen, S. E., Nelson, S. M., & Dosenbach, N. U. F. (2022). Individualized functional subnetworks connect human striatum and frontal cortex. Cerebral Cortex, 32(13), 2868–2884. https://doi.org/10.1093/cercor/bhab387.
Halcomb, M. E., Chumin, E. J., Goñi, J., Dzemidzic, M., & Yoder, K. K. (2019). Aberrations of anterior insular cortex functional connectivity in nontreatment-seeking alcoholics. Psychiatry Research: Neuroimaging, 284, 21–28.
U.S. Deptartment of Health and Human Services. (2014). The health consequences of smoking—50 years of progress: A report of the Surgeon General. Atlanta: US Department of Health and Human Services, Centers for Disease Control and Prevention, National Center for Chronic Disease Prevention and Health Promotion, Office on Smoking and Health.
Heimer, L., Alheid, G. F., de Olmos, J. S., Groenewegen, H. J., Haber, S. N., Harlan, R. E., & Zahm, D. S. (1997). The accumbens: Beyond the core-shell dichotomy. Journal of Neuropsychiatry and Clinical Neurosciences, 9(3), 354–381. https://doi.org/10.1176/jnp.9.3.354.
Janes, A. C., Nickerson, L. D., Frederick, B., & Kaufman, M. J. (2012). Prefrontal and limbic resting state brain network functional connectivity differs between nicotine-dependent smokers and non-smoking controls. Drug and Alcohol Dependence, 125(3), 252–259.
Kareken, D. A., Dzemidzic, M., Wetherill, L., Eiler, W. 2nd, Oberlin, B. G., Harezlak, J., Wang, Y., & O’Connor, S. J. (2013). Family history of alcoholism interacts with alcohol to affect brain regions involved in behavioral inhibition. Psychopharmacology (Berl), 228(2), 335–345. https://doi.org/10.1007/s00213-013-3038-4.
Korponay, C., Stein, E. A., & Ross, T. J. (2022). Dissociable misconfigurations of striatal functional connectivity profiles in smokers. bioRxiv, 2022.2002. 2015.480576.
Li, J., & Daw, N. D. (2011). Signals in human striatum are appropriate for policy update rather than value prediction. Journal of Neuroscience, 31(14), 5504–5511.
Lindquist, M. A., Geuter, S., Wager, T. D., & Caffo, B. S. (2019). Modular preprocessing pipelines can reintroduce artifacts into fMRI data. Human Brain Mapping, 40(8), 2358–2376.
Menon, V. (2011). Large-scale brain networks and psychopathology: A unifying triple network model. Trends in Cognitive Sciences, 15(10), 483–506. https://doi.org/10.1016/j.tics.2011.08.003.
Meredith, G. E., Pattiselanno, A., Groenewegen, H. J., & Haber, S. N. (1996). Shell and core in monkey and human nucleus accumbens identified with antibodies to calbindin-D28k. The Journal of Comparative Neurology, 365(4), 628–639. https://doi.org/10.1002/(SICI)1096-9861(19960219)365:43C;628::AID-CNE93E;3.0.CO;2-6.
Meyerhoff, D. J., Tizabi, Y., Staley, J. K., Durazzo, T. C., Glass, J. M., & Nixon, S. J. (2006). Smoking comorbidity in alcoholism: Neurobiological and neurocognitive consequences. Alcoholism, Clinical and Experimental Research, 30(2), 253–264. http://www.ncbi.nlm.nih.gov/entrez/query.fcgi?cmd=Retrieve&db=PubMed&dopt=Citation&list_uids=16441274.
Morales, A. M., Stark, N. A., & Nagel, B. J. (2021). Ventral striatal resting-state functional connectivity in adolescents is associated with earlier onset of binge drinking. Drug and Alcohol Dependence, 227, 109010.
Muschelli, J., Nebel, M. B., Caffo, B. S., Barber, A. D., Pekar, J. J., & Mostofsky, S. H. (2014). Reduction of motion-related artifacts in resting state fMRI using aCompCor. Neuroimage, 96, 22–35.
Naqvi, N. H., Gaznick, N., Tranel, D., & Bechara, A. (2014). The insula: A critical neural substrate for craving and drug seeking under conflict and risk. Annals of the New York Academy of Sciences, 1316(1), 53–70.
Ng, R., Sutradhar, R., Yao, Z., Wodchis, W. P., & Rosella, L. C. (2020). Smoking, drinking, diet and physical activity—modifiable lifestyle risk factors and their associations with age to first chronic disease. International Journal of Epidemiology, 49(1), 113–130.
Nickerson, L. D., Smith, S. M., Öngür, D., & Beckmann, C. F. (2017). Using dual regression to investigate network shape and amplitude in functional connectivity analyses. Frontiers in Neuroscience, 11, 115.
Oberlin, B. G., Dzemidzic, M., Tran, S. M., Soeurt, C. M., O’Connor, S. J., Yoder, K. K., & Kareken, D. A. (2015). Beer self-administration provokes lateralized nucleus accumbens dopamine release in male heavy drinkers. Psychopharmacology (Berl), 232(5), 861–870. https://doi.org/10.1007/s00213-014-3720-1.
Phạm, D. Đ., McDonald, D. J., Ding, L., Nebel, M. B., & Mejia, A. F. (2023). Less is more: Balancing noise reduction and data retention in fMRI with data-driven scrubbing. Neuroimage, 270, 119972.
Power, J. D., Mitra, A., Laumann, T. O., Snyder, A. Z., Schlaggar, B. L., & Petersen, S. E. (2014). Methods to detect, characterize, and remove motion artifact in resting state fMRI. Neuroimage, 84, 320–341.
Prensa, L., Richard, S., & Parent, A. (2003). Chemical anatomy of the human ventral striatum and adjacent basal forebrain structures. The Journal of Comparative Neurology, 460(3), 345–367. https://doi.org/10.1002/cne.10627.
Pruim, R. H., Mennes, M., van Rooij, D., Llera, A., Buitelaar, J. K., & Beckmann, C. F. (2015). ICA-AROMA: A robust ICA-based strategy for removing motion artifacts from fMRI data. Neuroimage, 112, 267–277.
Room, R. (2004). Smoking and drinking as complementary behaviours. Biomedicine & Pharmacotherapy, 58(2), 111–115. http://www.ncbi.nlm.nih.gov/entrez/query.fcgi?cmd=Retrieve&db=PubMed&dopt=Citation&list_uids=14992792.
Sazdanovic, M., Sazdanovic, P., Zivanovic-Macuzic, I., Jakovljevic, V., Jeremic, D., Peljto, A., & Tosevski, J. (2011). Neurons of human nucleus accumbens. Vojnosanitetski Pregled, 68(8), 655–660. https://doi.org/10.2298/vsp1108655s.
Shen, Z., Huang, P., Qian, W., Wang, C., Yu, H., Yang, Y., & Zhang, M. (2016). Severity of dependence modulates smokers’ functional connectivity in the reward circuit: A preliminary study. Psychopharmacology (Berl), 233, 2129–2137.
Shirer, W. R., Jiang, H., Price, C. M., Ng, B., & Greicius, M. D. (2015). Optimization of rs-fMRI pre-processing for enhanced signal-noise separation, test-retest reliability, and group discrimination. Neuroimage, 117, 67–79.
Smith, S. M., Beckmann, C. F., Andersson, J., Auerbach, E. J., Bijsterbosch, J., Douaud, G., Duff, E., Feinberg, D. A., Griffanti, L., & Harms, M. P. (2013). Resting-state fMRI in the human connectome project. Neuroimage, 80, 144–168.
Sobell, L. C., Brown, J., Leo, G. I., & Sobell, M. B. (1996). The reliability of the Alcohol Timeline Followback when administered by telephone and by computer. Drug and Alcohol Dependence, 42(1), 49–54.
Stolerman, I. P., & Jarvis, M. (1995). The scientific case that nicotine is addictive. Psychopharmacology (Berl), 117, 2–10.
Sullivan, J. T., Sykora, K., Schneiderman, J., Naranjo, C. A., & Sellers, E. M. (1989). Assessment of alcohol withdrawal: The revised clinical institute withdrawal assessment for alcohol scale (CIWA-Ar). British Journal of Addiction, 84(11), 1353–1357.
Tian, Y., Margulies, D. S., Breakspear, M., & Zalesky, A. (2020). Topographic organization of the human subcortex unveiled with functional connectivity gradients. Nature Neuroscience, 23(11), 1421–1432.
Van Skike, C. E., Maggio, S. E., Reynolds, A. R., Casey, E. M., Bardo, M. T., Dwoskin, L. P., Prendergast, M. A., & Nixon, K. (2016). Critical needs in drug discovery for cessation of alcohol and nicotine polysubstance abuse. Progress in Neuropsychopharmacology and Biological Psychiatry, 65, 269–287. https://doi.org/10.1016/j.pnpbp.2015.11.004.
Vergara, V. M., Liu, J., Claus, E. D., Hutchison, K., & Calhoun, V. (2017). Alterations of resting state functional network connectivity in the brain of nicotine and alcohol users. Neuroimage, 151, 45–54.
Voorn, P., Brady, L. S., Schotte, A., Berendse, H. W., & Richfield, E. K. (1994). Evidence for two neurochemical divisions in the human nucleus accumbens. European Journal of Neuroscience, 6(12), 1913–1916. https://doi.org/10.1111/j.1460-9568.1994.tb00582.x.
Voorn, P., Brady, L. S., Berendse, H. W., & Richfield, E. K. (1996). Densitometrical analysis of opioid receptor ligand binding in the human striatum–I. distribution of mu opioid receptor defines shell and core of the ventral striatum. Neuroscience, 75(3), 777–792. https://doi.org/10.1016/0306-4522(96)00271-0.
Wang, J. J., Durazzo, T. C., Gazdzinski, S., Yeh, P. H., Mon, A., & Meyerhoff, D. J. (2009). MRSI and DTI: A multimodal approach for improved detection of white matter abnormalities in alcohol and nicotine dependence. Nmr in Biomedicine, 22(5), 516–522. https://doi.org/10.1002/nbm.1363.
Whiteside, S. P., & Lynam, D. R. (2003). Understanding the role of impulsivity and externalizing psychopathology in alcohol abuse: Application of the UPPS impulsive behavior scale. Experimental and Clinical Psychopharmacology, 11(3), 210.
Xia, X., Fan, L., Cheng, C., Eickhoff, S. B., Chen, J., Li, H., & Jiang, T. (2017). Multimodal connectivity-based parcellation reveals a shell-core dichotomy of the human nucleus accumbens. Human Brain Mapping, 38(8), 3878–3898. https://doi.org/10.1002/hbm.23636.
Yoder, K. K., Albrecht, D. S., Dzemidzic, M., Normandin, M. D., Federici, L. M., Graves, T., Herring, C. M., Hile, K. L., Walters, J. W., Liang, T., Plawecki, M. H., O’Connor, S., & Kareken, D. A. (2016). Differences in IV alcohol-induced dopamine release in the ventral striatum of social drinkers and nontreatment-seeking alcoholics. Drug and Alcohol Dependence, 160, 163–169. https://doi.org/10.1016/j.drugalcdep.2016.01.001.
Zahm, D. S. (1999). Functional-anatomical implications of the nucleus accumbens core and shell subterritories. Annals of the New York Academy of Sciences, 877, 113–128. https://doi.org/10.1111/j.1749-6632.1999.tb09264.x.
Zhao, X., Yang, R., Wang, K., Zhang, Z., Wang, J., Tan, X., Zhang, J., Mei, Y., Chan, Q., Xu, J., Feng, Q., & Xu, Y. (2018). Connectivity-based parcellation of the nucleus accumbens into core and shell portions for stereotactic target localization and alterations in each NAc subdivision in mTLE patients. Human Brain Mapping, 39(3), 1232–1245. https://doi.org/10.1002/hbm.23912.
Zhou, S., Xiao, D., Peng, P., Wang, S. K., Liu, Z., Qin, H. Y., Li, S. S., & Wang, C. (2017). Effect of smoking on resting-state functional connectivity in smokers: An fMRI study. Respirology, 22(6), 1118–1124. https://doi.org/10.1111/resp.13048.
Acknowledgements
We would like to thank the Indiana Institute of Biomedical Imaging Sciences (IIBIS) In-Vivo Imaging Core, Dr. Yu-Chien Wu and Dr. Qiuting Wen for assistance with sequence development and testing. We also greatly appreciate MRI technologists Robert Bryant, Traci Day, and Will Korst for their help during imaging sessions. In addition, we would like to acknowledge Dr. Jenya Chumin’s excellent work in establishing the image processing protocol and assistance in study day activities.
Funding
This work was supported by the National Institute of Alcohol Abuse and Alcoholism grants R01 AA01835402 (KKY), R21 AA02265002 (KKY), and T32 AA00746532 (MEH).
Author information
Authors and Affiliations
Contributions
Author contributions included conception and study design (TCD, KKY), data collection, acquisition, and/or processing (MD, MEH, KLH, AAK), statistical analysis (MD, MEH, KKY), interpretation of results (TCD, MD, MEH, KKY), drafting the manuscript work or revising it critically for important intellectual content (MD, TCD, MEH, KKY) and approval of final version to be published and agreement to be accountable for the integrity and accuracy of all aspects of the work (All authors).
Corresponding author
Ethics declarations
Ethics approval
This study was approved by the Indiana University Institutional Review Board.
Consent to participate
All participants provided written informed consent.
Consent for publication
All authors consented to submit this work for publication.
Competing interests
The authors declare no competing interests.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Halcomb, M.E., Dzemidzic, M., Avena-Koenigsberger, A. et al. Greater ventral striatal functional connectivity in cigarette smokers relative to non-smokers across a spectrum of alcohol consumption. Brain Imaging and Behavior 18, 1121–1130 (2024). https://doi.org/10.1007/s11682-024-00903-9
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11682-024-00903-9