Abstract
Older age is associated with worsened outcome after mild traumatic brain injury (mTBI) and a higher risk of developing persistent post-traumatic complaints. However, the effects of mTBI sequelae on brain connectivity at older age and their association with post-traumatic complaints remain understudied.
We analyzed multi-echo resting-state functional magnetic resonance imaging data from 25 older adults with mTBI (mean age: 68 years, SD: 5 years) in the subacute phase (mean injury to scan interval: 38 days, SD: 9 days) and 20 age-matched controls. Severity of complaints (e.g. fatigue, dizziness) was assessed using self-reported questionnaires. Group independent component analysis was used to identify intrinsic connectivity networks (ICNs). The effects of group and severity of complaints on ICNs were assessed using spatial maps intensity (SMI) as a measure of within-network connectivity, and (static) functional network connectivity (FNC) as a measure of between-network connectivity.
Patients indicated a higher total severity of complaints than controls. Regarding SMI measures, we observed hyperconnectivity in left-mid temporal gyrus (cognitive-language network) and hypoconnectivity in the right-fusiform gyrus (visual-cerebellar network) that were associated with group. Additionally, we found interaction effects for SMI between severity of complaints and group in the visual(-cerebellar) domain. Regarding FNC measures, no significant effects were found.
In older adults, changes in cognitive-language and visual(-cerebellar) networks are related to mTBI. Additionally, group-dependent associations between connectivity within visual(-cerebellar) networks and severity of complaints might indicate post-injury (mal)adaptive mechanisms, which could partly explain post-traumatic complaints (such as dizziness and balance disorders) that are common in older adults during the subacute phase.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Effects of Mild Traumatic Brain Injury on Resting State Brain Network Connectivity in Older Adults
Mild traumatic brain injury (mTBI) is a leading public health issue worldwide (Carroll et al. 2014; Levin and Diaz-Arrastia 2015). Old and young adults are at highest risk of sustaining an mTBI (Bruns and Hauser 2003). With the world’s population increasing and ageing, the older population with mTBI will remain a growing group in the years to come (Roozenbeek et al. 2013). Self-reported cognitive, physical and affective complaints are common after mTBI and usually resolve within a few weeks. However, a subgroup experiences post-traumatic complaints (PTCs) that persist for months or years, which is often referred to as post-concussive syndrome (PCS; Levin and Diaz-Arrastia 2015). Older adults are more likely to experience slower recovery trajectories and persisting PTCs than their younger counterparts (King 2014). Nonetheless, little is known so far about the mechanisms behind the development of persisting PTCs at older age (Carroll et al. 2004; Kristman et al. 2014).
In terms of neurophysiology, TBI can cause diffuse axonal injury and focal brain damage (Johnson et al. 2013). Although growing evidence supports that damage might occur even if injury severity is mild, this cannot be detected using conventional neuroimaging techniques (Sharp et al. 2014). Functional magnetic resonance imaging (fMRI) is an advanced neuroimaging technique that allows the investigation of brain networks linked to various domains of functioning (e.g. cognition, vision). Particularly, resting state fMRI (rs-fMRI) is a suitable modality to study how aging and several neuropathologies, such as TBI, affect the brain. In healthy participants, consistent patterns for resting state networks connectivity have been found (see Smith et al. (2013) and van den Heuvel and Hulshoff Pol (2010) for reviews). Thus, investigating altered patterns of brain network connectivity can provide insight on how aging and mTBI might affect the brain and the development of complaints after brain injury. Previous studies have investigated brain network connectivity alterations at older age (vs. younger age) (Geerligs et al. 2015), during healthy aging (Allen et al. 2011), after mTBI (see Rosenthal et al. (2018) and Sharp et al., (2014) for reviews) and the interaction between mTBI and aging during adulthood (Bittencourt-Villalpando et al. 2021). With ageing, ICNs throughout the brain undergo profound changes in their connectivity patterns. This reorganization process results in weaker long-distance between-network connectivity and stronger short-distance between-network connectivity (Damoiseaux 2017). Besides, ICNs that are involved in high-order cognitive processes become less specialized and less differentiated from each other (Geerligs et al. 2015). This phenomenon is thought to be part of (attempted) compensatory mechanisms for sustaining cognitive performance by recruiting additional neighboring neural resources, which may be successful or not (Grady 2012).
By contrast, the effects of mTBI on brain connectivity are less clear. Abnormalities later than one month post-injury are thought to be associated with (the development of) PCS and/or neuropsychiatric conditions (Rosenthal et al. 2018; Stevens et al. 2012; van der Horn et al. 2016). Furthermore, aging is an important confounder in mTBI studies. Notwithstanding the effects of aging in brain connectivity are stronger than those of mTBI, most studies do not address age in their analysis beyond age-matching patient and control groups (Bittencourt-Villalpando et al., 2021). To date, the associations between the development of PTCs and alterations in brain connectivity are unclear and little is known about the effects of mTBI on brain connectivity in older adults.
The current study is the first to investigate brain network connectivity specifically in older adults with mTBI (OA-mTBI; age ≥ 60 years old). Here, we analyzed within- and between-network connectivity of intrinsic connectivity networks (ICNs), following a similar multivariate approach as previously used by our research group to investigate the effects of ageing and mTBI in a different adult population (age range: 18–65 years, Bittencourt-Villalpando et al., 2021). It is known that ageing affects both between- and within-network connectivity (Damoiseaux 2017; Geerligs et al. 2015). Here, we hypothesize that mTBI acts as a stressor on brain functioning and that, at older age, fewer resources are available to cope with the injury. In light of our recent work (Bittencourt-Villalpando et al. 2021), we expect that the most prominent age- and/or mTBI-related effects on brain network connectivity would involve cognitive, default mode, and/or cerebellar networks, and that perturbations of these networks might underlie persistent complaints in older adults with mTBI. Therefore, an additional research aim was to explore possible associations between severity of (post-traumatic) complaints with altered rs-fMRI brain network connectivity.
Methods
Participants
Data from OA-mTBI (age > 60 years) and age-matched (older adults) healthy controls (OA-HC) were obtained as part of a larger prospective follow-up study (ReCONNECT-study). Patients were included at the University Medical Center Groningen (UMCG), the Netherlands (a level 1 trauma center) between November 2018 and November 2020. The diagnosis of mTBI was based on the following criteria: attending the hospital with an mTBI defined by a Glasgow Coma Scale score of 13–15, loss of consciousness ≤ 30 min and/or post-traumatic amnesia of ≤ 24 h (Kay et al. 1993). Inclusion criteria were comprehension of the Dutch language and age 60 years or older. Healthy controls were recruited via social contacts and advertisements. For both groups, exclusion criteria were: a history of drug or alcohol abuse, a major psychiatric or neurologic disorder (e.g. schizophrenia, bipolar disorder, major depressive disorder, and dementia) as previously diagnosed or identified by the attending or ward physician and/or a previous hospital admission due to a TBI. The ReCONNECT-study was approved by the Medical Ethical Committee of the UMCG; written informed consent was obtained from all participants. All procedures were performed according to the declaration of Helsinki (2013).
Current and Pre-Injury Severity of Complaints
The Head Injury Symptoms Checklist (HISC) is a 21-item post-traumatic questionnaire (de Koning et al. 2016), derived from the Rivermead Post-concussion Symptoms Questionnaire (King et al. 1995). The HISC was administered to all participants on the day of the fMRI scan. One of the 21 complaints, namely intolerance to alcohol, was excluded from the analysis as most patients left a remark on the questionnaire explaining that they did not consume alcohol since the injury. The HISC scores the current and the pre-injury severity level of each complaint with values ranging from 0 to 2 (never = 0, sometimes = 1, and often = 2). For OA-HCs, only current severity level of complaints score (HISC-sev) can be assessed. The HISC-sev was then calculated as the sum of the severity of all the 20 current complaints assessed (ranging from 0 to 40). For OA-mTBI, additionally, post-traumatic symptoms were defined as new complaints or complaints that were increased after mTBI (i.e. a positive result after the subtraction of pre-injury from current scores, for each complaint).
Only current severity level of complaints score (HISC-sev) was used in further statistical analysis. The HISC-sev scores were first square root (sqrt)-transformed to achieve a normal distribution (sqrt(HISC)).
FMRI Acquisition
A Siemens MAGNETOM Prisma 3 T MRI scanner (Siemens, Erlangen, Germany) equipped with a 64-channel SENSE head coil was used for image acquisition. A high-resolution transversal T1-weighted image was made for anatomical reference (repetition time [TR] 2300 ms; echo-time [TE] 2.98 ms; flip angle [FA] 9°; field of view [FOV] 256 × 240 × 176 mm; voxel size 1 × 1 × 1.2 mm). For resting-state imaging, T2*-weighted echo planar imaging volumes were acquired with a multi-echo-EPI sequence (TR 2000 ms, TE 9.74, 22.10 and 34.46 ms; FA = 60°; FOV 256 × 256 mm; voxel size 3.5 mm-isotropic) (Feinberg et al. 2010; Moeller et al. 2010; Xu et al. 2013).
Patient rs-fMRI data were acquired at approximately five weeks after injury. All participants were instructed to close their eyes and to stay awake (duration: 10 min, 300 volumes).
FMRI Preprocessing
First, multi-echo ICA (ME-ICA; meica.py script version 3.2; Kundu et al. 2012, 2017) was applied for denoising and image realignment to the first functional image. Afterwards, Statistical Parametric Mapping (SPM12, http://www.fil.ion.ucl.ac.uk/spm/, Ashburner & Friston, 2005) implemented in Matlab (version R2020a; MathWorks, Natick, MA) was used. The SPM preprocessing pipeline consisted of co-registration of functional images with individual participants’ T1-weighted images and normalization to the Montreal Neurological Institute template using a diffeomorphic nonlinear registration tool (DARTEL; isotropic voxels of 3 mm; smoothing 6 mm full-width-at-half-maximum Gaussian kernel). The first five volumes from each participant were excluded from the analysis for T1 equilibration.
Group ICA
We used Group ICA fMRI toolbox (GIFT; version 4.0c; Calhoun et al. 2001) for the identification of ICNs. This approach is described in the Appendix.
Statistical Analysis
For the set of C identified ICNs, we assessed two measures of functional connectivity: spatial map intensity (SMI) and (static) functional network connectivity (FNC) as measures of within- and between-network connectivity, respectively.
The design matrix included four covariates of interest: group as a categorical variable (OA-mTBI or OA-HC), severity of complaints score (i.e. HISC-sev) as a continuous variable, age as a continuous variable (because it has been identified as a strong confounder for mTBI effects in mid-adulthood (Bittencourt-Villalpando et al., 2021)) and the interaction term group × HISC-sev. Additionally, we included average framewise displacement (FD; in mm; Power et al. 2012) as a nuisance covariate to control for head motion. FD was first dichotomized into a categorical variable (high- and low-movement) using median split due to its skewed distribution.
We followed a multivariate approach as proposed by (Allen et al., 2011) using the MANCOVAN toolbox, which is implemented in GIFT. The MANCOVAN implementation tests the explained variance of each covariate for each of the functional connectivity measures using multivariate analysis of covariance and performs backward selection of the model terms. The purpose of this procedure is to select important covariates before performing the univariate tests. For more details on the MANCOVAN implementation, please refer to Allen et al., (2011).
For SMI results, in case of main or interaction effects for the covariates of interest, z-scores were averaged over all significant voxels exhibiting significant effects of the same sign (positive or negative). The results were visualized using boxplots.
We calculated the Pearson correlation coefficients (r) to measure the strength of the linear relationship between continuous variables of interest (e.g. sqrt(HISC)) and the dependent variables using the “corr” function implemented in Matlab. The results were visualized using scatter plots.
Testing for group differences was done in IBM SPSS Statistics, Version 26.0 using independent T-tests for normally distributed continuous variables and Pearson Chi-square tests for categorical variables.
All tests were corrected for multiple comparisons at an α = 0.05 significance level using false discovery rate (FDR; Genovese et al. 2002) correction.
Post-hoc Analysis
We performed an additional post-hoc analysis to verify the association between complaints reporting (per complaint domains) and altered brain connectivity. First, complaint domains were determined according to a modularity analysis using HISC data that were obtained from a larger mTBI population (UPFRONT study; van der Naalt et al. 2017). The post-hoc analysis is detailed in the Appendix.
Results
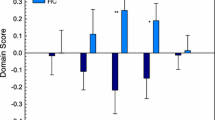
The summary of participant characteristics per group is presented in Table 1. We did not identify any significant differences for age, sex, nor FD between groups. Older adults with mTBI had higher (current) severity of complaints than OA-HC (p < 0.001). Additionally, pre-injury severity complaints scores reported by OA-mTBI were similar to current severity complaints scores reported by OA-HCs (p = 0.47). An overview of the prevalence of individual complaints per group (OA-mTBI or OA-HC) is presented in Fig. 1. The prevalence of post-traumatic symptoms within the OA-mTBI group can be found in the Appendix, Fig. S1.
Overview of the prevalence of complaints (%) per group and per domain (OA-mTBI: older adults with mTBI; OA-HC: older adults-healthy controls; DOM: complaint domain). Complaints ordered by prevalence in the older adults with mTBI group from highest to lowest within each of the five identified complaint domains. Asterisks indicated significant differences between groups (Chi-square tests; *p < 0.05, FDR-corrected; †p < 0.05, uncorrected)
Group ICA and Statistical Analysis
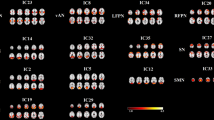
The group ICA resulted in C = 15 ICNs, which were grouped into five functional domains, one of which defined as a mixed domain (i.e. ICNs with high activation in more than one functional domain) (see Appendix, Fig. S2).
The results from the multivariate tests representing the significance of the covariates of interest in predicting SMI and FNC for the 15 identified ICNs are shown in the Appendix, Fig. S3. None of the covariates of interest were retained as predictors for FNC.
Subsequently, we identified for which ICN regions (voxels) the SMI were associated with the retained covariates of interest.
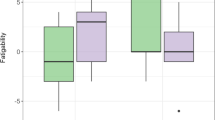
The main effects of group on SMI are shown in Fig. 2. In comparison to healthy counterparts, OA-mTBI showed increased SMI in the left middle temporal gyrus (lMTG)(ICN13; Cog-C/Lan; t = -6.69, p < 0.001, V = 378mm3; Fig. 2A-B) and decreased SMI in the right posterior fusiform gyrus (ICN5; Vis-CB; t = 5.07, p < 0.001, V = 108mm3, Fig. 2C-D).
Effects of group on spatial map intensity (SMI). (A, C): Significant effect of group (older adults with mild traumatic brain injury; OA-mTBI vs. older adults-Healthy controls; OA-HCs) for SMI of intrinsic connectivity network (ICN) showing increased SMI for OA-mTBI in the left middle temporal gyrus on ICN13, and decreased SMI for OA-mTBI in the right posterior fusiform gyrus on ICN5 (p < 0.05, FDR-corrected) in representative slices. (B, D): Boxplots of the SMI averaged over all significant voxels across participants, per group. Cog-C/Lan: Cognitive-language domain; Vis-CB: Visual(-cerebellar) domain
Interaction effects of sqrt(HISC) × group on SMI are shown in Fig. 3. In the anterior fusiform and middle occipital gyri (ICN7; Vis-CB), SMI decreased with sqrt(HISC) in OA-HC, but increased with sqrt(HISC) in OA-mTBI (OA-HC: r = -0.80; p < 0.001; OA-mTBI: r = -0.87, p < 0.001; V = 1512mm3; Fig. 3A-B).
Group × sqrt(HISC) interaction effect on spatial map intensity (SMI) of older adults with mild traumatic brain injury (OA-mTBI) and older adults HCs (OA-HCs). (A, C, E): Voxels with significant Group × sqrt(HISC) interaction effect for SMI of intrinsic connectivity network (ICN) in the anterior fusiform and middle occipital gyri on ICN7 (A), in the cerebellum VI and Crus I bilaterally on ICN7 (C) and in the cuneus on ICN4 (E) (p < 0.05, FDR-corrected). (B, D, F): Scatterplot of sqrt(HISC) against SMI (z-score) averaged over all significant voxels across participants. Blue: OA-HCs; red: OA-mTBI; sqrt(HISC): square root transformed severity of complaints score. Vis-CB: Visual(-cerebellar) domain
In the cerebellum VI and Crus I bilaterally (ICN7; Vis-CB), SMI increased with sqrt(HISC) in OA-HC, but decreased with sqrt(HISC) in OA-mTBI (OA-HC: r = 0.75; p < 0.001; OA-mTBI: r = -0.61; p = 0.001; V = 594mm3; Fig. 3C-D).
In the cuneus ICN4(Vis-CB), SMI decreased with sqrt(HISC) in OA-HC, but increased with sqrt(HISC) in OA-mTBI (OA-HC: r = -0.64; p < 0.01; OA-mTBI: r = 0.74, p < 0.001; V = 324mm3; Fig. 3E-F).
Post-hoc Analysis
The results of the post-hoc analysis can be found in the Appendix.
Discussion
In this study, we examined fMRI-ICNs in a sample of OA-mTBI and age-matched OA-HCs. We employed an ICA-based multivariate approach to identify the effects of mTBI on brain network connectivity, severity of complaints and their interaction at older age. We identified main effects of mTBI on network connectivity within visual(-cerebellar) and cognitive-language networks. Additionally, we identified interaction effects between severity of complaints and group on network connectivity within visual(-cerebellar) networks, suggesting that these regions are the most affected in older adults that have sustained an mTBI. No effects on between-network connectivity were found. Our findings are discussed below.
We found increased within-network connectivity for OA-mTBI in comparison to OA-HCs in clusters located in the left middle temporal gyrus (lMTG) of the cognitive-language ICN. The lMTG is involved in language processing, which requires a complex integration of sensory inputs (e.g. visual and auditory) (Fridriksson et al. 2016; Hickok and Poeppel 2007). Hyperconnectivity is a common finding after TBI, but its adaptive or maladaptive nature remains unclear (Hillary and Grafman 2017; Morelli et al. 2021). Previous mTBI studies using task-fMRI in younger cohorts found that hyperconnectivity in functional networks involved in attentional and visual processes might be associated with increased subjective effort and task-related fatigue (Prak et al. 2021). In our cohort, fatigue (together with dizziness) was the most prevalent complaint after mTBI, being present in 60% of the OA-mTBI (see Fig. 1). Additionally, in our post-hoc analysis we found that connectivity within the lMTG increased with severity of complaints in both cognitive-fatigue and vestibular domains, which includes noise intolerance complaints (see Appendix, Table S1). Perhaps increased awareness of external sensory stimuli could partly explain increased fatigue that is commonly experienced in elderly after mTBI.
Additionally, we found decreased within-network connectivity for OA-mTBI in comparison to OA-HCs in a cluster located posteriorly in the right fusiform gyrus (rFG) of a visual-cerebellar ICN. The rFG, particularly, is known for its role in visual pattern recognition (Grill-Spector et al. 2006), including facial recognition (Kanwisher et al. 1997). Our findings are consistent with previous studies that identified hypoactivity within visual networks of patients in the (sub)acute phase after mTBI (Amir et al. 2021; Stevens et al. 2012). Moreover, it has been suggested that the subacute phase after mTBI is marked by hypoactivation in several (non-DMN) areas across the brain, which normalizes in those patients who show good recovery (Bharath et al. 2015; Rosenthal et al. 2018). We encourage future research to investigate the longitudinal effects of mTBI on brain connectivity in the visual cortex.
In this study, we found interactions between the severity of complaints and group (OA-HC and OA-mTBI) for within-network connectivity. In clusters anteriorly located in the fusiform gyri (FG), in the middle occipital gyri and in the cuneus of two visual(-cerebellar) ICNs, within-network connectivity increased with severity of complaints score in OA-mTBI, but decreased with severity of complaints in OA-HCs. The anterior FG and the middle occipital gyri are involved in both semantic and visual processing whereas the cuneus is part of the primary visual cortex. Furthermore, in clusters located in the cerebellum bilaterally, within-network connectivity and severity of complaints score were negatively correlated in OA-mTBI, but positively correlated in OA-HCs. These results might reflect a difference in the association between brain functioning and (a high number of/more severe) complaints that are new or have recently increased in severity after a traumatic event (i.e. mTBI) and the association between (a low number of/less severe) complaints that people have been dealing with for a longer time (OA-HC). Interestingly, the cerebellum seems to play a key role in our findings. This might not be surprising considering the importance of this brain structure in integration of sensory, motor, cognitive and emotional information (Buckner 2013; Schmahmann 2019). It could be hypothesized that mTBI in older adults especially affects those networks that are responsible for multimodal integration. This is an interesting avenue for further research.
The question is how our findings can be related to the functional role of the cerebellum and of the visual cortex in light of previous research. Previous studies identified an association between PTCs and altered brain connectivity after mTBI (see van der Horn et al. 2016 and Biagianti et al. 2020 for reviews). A few of these studies identified connectivity within visual and/or cerebellar areas among those associated with PTCs (Nathan et al. 2015; Palacios et al. 2017; Stevens et al. 2012), although no clear pattern for their association emerged. It is known that, after mTBI, vision impairment including blurred vision and altered oculo-vestibular reflexes are common, although the etiology of these symptoms is still not well understood (Fife and Kalra 2015). In our OA-mTBI cohort, dizziness was the most prevalent self-reported symptom and complaint (see Fig. 1 and Appendix, Fig. S1) and complaints of balance disorders were (significantly) higher than in the OA-HC group, indicating that vestibular impairments might have been present during the subacute phase after trauma. It is therefore tempting to suggest a possible association of activity in the visual(-cerebellar) domain with vestibular impairments after mTBI. Perhaps the observed increasing hyperactivation in the visual cortex of OA-mTBI with severity of complaints score indicates increased effort to compensate for functional deficits, including oculo-vestibular impairments. However, as we do not have a direct association of vestibular and/or visual functioning measures with brain activity, such suggestion is speculative. Nevertheless, in our post-hoc analysis we found that, in OA-mTBI, connectivity within cerebellar areas decreased with severity of complaints in the vestibular domain, whereas connectivity within visual areas (i.e. anterior FG, middle occipital gyri and cuneus) increased with severity of complaints in the vestibular domain, among other domains (see Appendix, Table S1). Future studies are required to verify if a direct association of hypoactivation in the cerebellar area with vestibular impairments in OA-mTBI exists and elucidate if (attempted) compensation via increased activation in the visual cortex is part of this scene.
Regarding between-network connectivity (i.e. FNC), we did not find any effects for age or mTBI. In a previous study of our research group where we used a similar approach to analyze a younger mTBI population, we found that age strongly affected between-network connectivity during mid-adulthood, whereas no effects of mTBI were found (Bittencourt-Villalpando et al. 2021). Of note, patient and control groups were age-matched in both studies. We will discuss mTBI- and age- related findings on between-network connectivity in this order. First, there is still no consensus in the literature regarding the effects of mTBI on between-network connectivity due to lack of consistency and repeatability across studies. By employing a similar methodology and robustly controlling for age-related effects, both of our studies were consistent in identifying no effect of mTBI on FNC, suggesting that sustaining an mTBI does not affect between-network connectivity (at rest) regardless of age. Furthermore, in both of our studies, we opted to scan patients at approximately five weeks after the injury, to explore abnormalities in brain connectivity that might be associated with persistent symptoms instead of short-lived ones. Since the optimal interval for this purpose remains unknown, it is possible that shorter or longer injury to scan intervals would lead to different results. Second, the lack of age-related effects on brain connectivity in the present study might be explained by the smaller age range of its population (i.e. 20 years instead of 47 years) and/or by larger variability in age-related effects at older age; additionally, it concurs with the notion that changes in brain network connectivity occur at a slower pace at older age.
To the best of our knowledge, this is the first study to investigate (whole-)brain network connectivity in OA-mTBI. Our approach for denoising using multi-echo ICA resulted in all ICs identified as ICNs (as opposed to artifacts). Nevertheless, this study entails some limitations. First, in older adults, co-existing visual deterioration (that generally occurs as part of the aging process; Chou et al. 2016) could partly contribute to altered connectivity in visual networks and motor-balance control. However, objective measures of pre- and post-injury visual and motor-balance functioning were not available, which limits our analysis. Second, although the fMRI scan environment is noisy and uncomfortable, it is possible that participants swerved in and out of light sleep states during the scan. Since we did not identify significant differences regarding sleep-related complaints between OA-mTBI and OA-HCs as assessed with the HISC, we do not expect differences between groups related to this factor. However, we acknowledge that we did not verify if participants fell asleep as a limitation of this study. Third, although we do not have a reason to suspect that there would be differences between groups regarding caffeine consumption and/or its (temporary) effects on blood flow, we did not control for this factor. Lastly, due to the cross-sectional nature of this study, it is unknown how the identified alterations on brain connectivity and their interaction with PTCs evolve over time. Longitudinal studies are required to identify whether the observed effects on brain connectivity and the interaction between altered connectivity and severity of complaints can be replicated and predict the persistence of complaints. This knowledge might help defining more effective rehabilitation strategies for older adults at risk of developing persistent PTCs.
Conclusions
Our findings on altered brain connectivity in OA-mTBI converged in visual(-cerebellar) and cognitive-language networks, some of which were associated with severity of complaints. These findings bring indirect evidence of a possible association of abnormal activation in brain networks with oculo-vestibular and cognitive impairments and warrant further investigation.
Data availability
Not applicable.
References
Allen, E., Erhardt, E., Damaraju, E., Gruner, W., Segall, J., Silva, R., et al. (2011). A Baseline for the Multivariate Comparison of Resting-State Networks . Frontiers in Systems Neuroscience . https://www.frontiersin.org/article/https://doi.org/10.3389/fnsys.2011.00002
Amir, J., Nair, J. K. R., Del Carpio-O’Donovan, R., Ptito, A., Chen, J. K., Chankowsky, J., et al. (2021). Atypical resting state functional connectivity in mild traumatic brain injury. Brain and Behavior. https://doi.org/10.1002/brb3.2261
Bharath, R. D., Munivenkatappa, A., Gohel, S., Panda, R., Saini, J., Rajeswaran, J., et al. (2015). Recovery of resting brain connectivity ensuing mild traumatic brain injury. Frontiers in Human Neuroscience, 9(September). https://doi.org/10.3389/fnhum.2015.00513
Biagianti, B., Stocchetti, N., Brambilla, P., & Van Vleet, T. (2020). Brain dysfunction underlying prolonged post-concussive syndrome: A systematic review. Journal of Affective Disorders. https://doi.org/10.1016/j.jad.2019.10.058
Bittencourt-Villalpando, M., van der Horn, H. J., Maurits, N. M., & van der Naalt, J. (2021). Disentangling the effects of age and mild traumatic brain injury on brain network connectivity: A resting state fMRI study. NeuroImage: Clinical, 29, 102534.
Bruns, J., & Hauser, W. A. (2003). The Epidemiology of Traumatic Brain Injury: A Review. Epilepsia. https://doi.org/10.1046/j.1528-1157.44.s10.3.x
Calhoun, V. D., Adali, T., Pearlson, G. D., & Pekar, J. J. (2001). A method for making group inferences from functional MRI data using independent component analysis. Human Brain Mapping. https://doi.org/10.1002/hbm.1048
Carroll, L. J., Cancelliere, C., Côté, P., Hincapié, C. A., Kristman, V. L., Holm, L. W., et al. (2014). Systematic Review of the Prognosis After Mild Traumatic Brain Injury in Adults: Cognitive, Psychiatric, and Mortality Outcomes: Results of the International Collaboration on Mild Traumatic Brain Injury Prognosis. Archives of Physical Medicine and Rehabilitation, 95(3), S152–S173. https://doi.org/10.1016/j.apmr.2013.08.300
Carroll, L. J., Cassidy, J. D., Peloso, P. M., Borg, J., von Holst, H., Holm, L., et al. (2004). Prognosis for mild traumatic brain injury: Results of the WHO Collaborating Centre Task Force on Mild Traumatic Brain Injury. Journal of Rehabilitation Medicine, Supplement. https://doi.org/10.1080/16501960410023859
Chou, R., Dana, T., Bougatsos, C., Grusing, S., & Blazina, I. (2016). Screening for impaired visual acuity in older adults: Updated evidence report and systematic review for the US preventive services task force. JAMA - Journal of the American Medical Association. https://doi.org/10.1001/jama.2016.0783
Damoiseaux, J. S. (2017). Effects of aging on functional and structural brain connectivity. NeuroImage, 160, 32–40. https://doi.org/10.1016/j.neuroimage.2017.01.077
de Koning, M. E., Gareb, B., El Moumni, M., Scheenen, M. E., van der Horn, H. J., Timmerman, M. E., et al. (2016). Subacute posttraumatic complaints and psychological distress in trauma patients with or without mild traumatic brain injury. Injury, 47(9), 2041–2047. https://doi.org/10.1016/j.injury.2016.04.036
Feinberg, D. A., Moeller, S., Smith, S. M., Auerbach, E., Ramanna, S., Glasser, M. F., et al. (2010). Multiplexed echo planar imaging for sub-second whole brain fmri and fast diffusion imaging. PLoS ONE. https://doi.org/10.1371/journal.pone.0015710
Fife, T. D., & Kalra, D. (2015). Persistent vertigo and dizziness after mild traumatic brain injury. Annals of the New York Academy of Sciences. https://doi.org/10.1111/nyas.12678
Fridriksson, J., Yourganov, G., Bonilha, L., Basilakos, A., Den Ouden, D. B., & Rorden, C. (2016). Revealing the dual streams of speech processing. Proceedings of the National Academy of Sciences of the United States of America, 113(52). https://doi.org/10.1073/pnas.1614038114
Geerligs, L., Maurits, N. M., Renken, R. J., & Lorist, M. M. (2014). Reduced specificity of functional connectivity in the aging brain during task performance. Human Brain Mapping. https://doi.org/10.1002/hbm.22175
Geerligs, L., Renken, R. J., Saliasi, E., Maurits, N. M., & Lorist, M. M. (2015). A Brain-Wide Study of Age-Related Changes in Functional Connectivity. Cerebral Cortex. https://doi.org/10.1093/cercor/bhu012
Genovese, C. R., Lazar, N. A., & Nichols, T. (2002). Thresholding of statistical maps in functional neuroimaging using the false discovery rate. NeuroImage. https://doi.org/10.1006/nimg.2001.1037
Grady, C. (2012). The cognitive neuroscience of ageing. Nature Reviews Neuroscience. https://doi.org/10.1038/nrn3256
Grill-Spector, K., Sayres, R., & Ress, D. (2006). High-resolution imaging reveals highly selective nonface clusters in the fusiform face area. Nature Neuroscience. https://doi.org/10.1038/nn1745
Hickok, G., & Poeppel, D. (2007). The cortical organization of speech processing. Nature Reviews Neuroscience. https://doi.org/10.1038/nrn2113
Hillary, F. G., & Grafman, J. H. (2017). Injured Brains and Adaptive Networks: The Benefits and Costs of Hyperconnectivity. Trends in Cognitive Sciences. https://doi.org/10.1016/j.tics.2017.03.003
Johnson, V. E., Stewart, W., & Smith, D. H. (2013). Axonal pathology in traumatic brain injury. Experimental Neurology. https://doi.org/10.1016/j.expneurol.2012.01.013
Kanwisher, N., McDermott, J., & Chun, M. M. (1997). The fusiform face area: A module in human extrastriate cortex specialized for face perception. Journal of Neuroscience. https://doi.org/10.1523/jneurosci.17-11-04302.1997
Kay, T., Harrington, D. E., Adams, R., Anderson, T., Berrol, S., Cicerone, K., et al. (1993). Definition of mild traumatic brain injury. Journal of Head Trauma Rehabilitation, 8(3). https://doi.org/10.1097/00001199-199309000-00010
King, N. S., Crawford, S., Wenden, F. J., Moss, N. E. G., & Wade, D. T. (1995). The Rivermead Post Concussion Symptoms Questionnaire: A measure of symptoms commonly experienced after head injury and its reliability. Journal of Neurology. https://doi.org/10.1007/BF00868811
King, N. S. (2014). A systematic review of age and gender factors in prolonged post-concussion symptoms after mild head injury. Brain injury, 28(13–14), 1639–1645. https://doi.org/10.3109/02699052.2014.954271
Kristman, V. L., Borg, J., Godbolt, A. K., Salmi, L. R., Cancelliere, C., Carroll, L. J., et al. (2014). Methodological issues and research recommendations for prognosis after mild traumatic brain injury: Results of the international collaboration on mild traumatic brain injury prognosis. Archives of Physical Medicine and Rehabilitation. https://doi.org/10.1016/j.apmr.2013.04.026
Kundu, P., Benson, B. E., Rosen, D., Frangou, S., Leibenluft, E., Luh, W. M., et al. (2018). The integration of functional brain activity from adolescence to adulthood. Journal of Neuroscience. https://doi.org/10.1523/JNEUROSCI.1864-17.2018
Kundu, P., Inati, S. J., Evans, J. W., Luh, W. M., & Bandettini, P. A. (2012). Differentiating BOLD and non-BOLD signals in fMRI time series using multi-echo EPI. NeuroImage. https://doi.org/10.1016/j.neuroimage.2011.12.028
Kundu, P., Voon, V., Balchandani, P., Lombardo, M. V., Poser, B. A., & Bandettini, P. A. (2017). Multi-echo fMRI: A review of applications in fMRI denoising and analysis of BOLD signals. NeuroImage. https://doi.org/10.1016/j.neuroimage.2017.03.033
Levin, H. S., & Diaz-Arrastia, R. R. (2015). Diagnosis, prognosis, and clinical management of mild traumatic brain injury. The Lancet Neurology. https://doi.org/10.1016/S1474-4422(15)00002-2
Moeller, S., Yacoub, E., Olman, C. A., Auerbach, E., Strupp, J., Harel, N., & Uǧurbil, K. (2010). Multiband multislice GE-EPI at 7 tesla, with 16-fold acceleration using partial parallel imaging with application to high spatial and temporal whole-brain FMRI. Magnetic Resonance in Medicine. https://doi.org/10.1002/mrm.22361
Morelli, N., Johnson, N. F., Kaiser, K., Andreatta, R. D., Heebner, N. R., & Hoch, M. C. (2021). Resting state functional connectivity responses post-mild traumatic brain injury: A systematic review. Brain Injury, 0(0), 1–12. https://doi.org/10.1080/02699052.2021.1972339
Nathan, D. E., Oakes, T. R., Yeh, P. H., French, L. M., Harper, J. F., Liu, W., et al. (2015). Exploring variations in functional connectivity of the resting state default mode network in mild traumatic brain injury. Brain Connectivity. https://doi.org/10.1089/brain.2014.0273
Palacios, E. M., Yuh, E. L., Chang, Y. S., Yue, J. K., Schnyer, D. M., Okonkwo, D. O., et al. (2017). Resting-state functional connectivity alterations associated with six-month outcomes in mild traumatic brain injury. Journal of Neurotrauma, 34(8). https://doi.org/10.1089/neu.2016.4752
Power, J. D., Barnes, K. A., Snyder, A. Z., Schlaggar, B. L., & Petersen, S. E. (2012). Spurious but systematic correlations in functional connectivity MRI networks arise from subject motion. NeuroImage. https://doi.org/10.1016/j.neuroimage.2011.10.018
Prak, R. F., Marsman, J.-B. C., Renken, R., van der Naalt, J., & Zijdewind, I. (2021). Fatigue following mild traumatic brain injury relates to visual processing and effort perception in the context of motor performance. NeuroImage: Clinical, 32, 102783. https://doi.org/10.1016/j.nicl.2021.102783
Roozenbeek, B., Maas, A. I. R., & Menon, D. K. (2013). Changing patterns in the epidemiology of traumatic brain injury. Nature Reviews Neurology. https://doi.org/10.1038/nrneurol.2013.22
Rosenthal, S., Gray, M., Fatima, H., Sair, H. I., & Whitlow, C. T. (2018). Functional MR Imaging: Blood Oxygen Level-Dependent and Resting State Techniques in Mild Traumatic Brain Injury. Neuroimaging Clinics of North America. https://doi.org/10.1016/j.nic.2017.09.008
Sharp, D. J., Scott, G., & Leech, R. (2014). Network dysfunction after traumatic brain injury. Nature Reviews Neurology. https://doi.org/10.1038/nrneurol.2014.15
Stevens, M. C., Lovejoy, D., Kim, J., Oakes, H., Kureshi, I., & Witt, S. T. (2012). Multiple resting state network functional connectivity abnormalities in mild traumatic brain injury. Brain Imaging and Behavior. https://doi.org/10.1007/s11682-012-9157-4
van der Horn, H. J., Liemburg, E. J., Scheenen, M. E., de Koning, M. E., Spikman, J. M., & van der Naalt, J. (2016). Post-concussive complaints after mild traumatic brain injury associated with altered brain networks during working memory performance. Brain Imaging and Behavior, 10(4), 1243–1253. https://doi.org/10.1007/s11682-015-9489-y
Xu, J., Moeller, S., Auerbach, E. J., Strupp, J., Smith, S. M., Feinberg, D. A., et al. (2013). Evaluation of slice accelerations using multiband echo planar imaging at 3T. NeuroImage. https://doi.org/10.1016/j.neuroimage.2013.07.055
Acknowledgements
The authors would like to thank Brigit Klever in the UMCG for her contribution to data collection and organization.
Funding
This study was partly funded by a personal grant from the Research School of Behavioural and Cognitive Neurosciences and the Graduate School of Medical Sciences of the University of Groningen (awarded to the first author).
Author information
Authors and Affiliations
Contributions
MB: Conceptualization, Methodology, Software, Formal analysis, Investigation, Writing—original draft. HJvdH: Conceptualization, Methodology, Writing-Reviewing and Editing. SABS: Investigation, Writing-Reviewing and Editing. JBCM: Software, Writing-Reviewing and Editing. JvdN: Supervision, Writing-Reviewing and Editing. NMM: Supervision, Writing-Reviewing and Editing.
Corresponding author
Ethics declarations
Ethical Approval
The ReCONNECT-study was approved by the Medical Ethical Committee of the UMCG; written informed consent was obtained from all participants. All procedures were performed according to the declaration of Helsinki (2013).
Consent to Participate
Written informed consent was obtained from all participants.
Consent to Publish
The manuscript has been read and approved for submission by all (co-)authors.
Competing Interests
None of the authors have a conflict of interest to declare.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Bittencourt, M., van der Horn, HJ., Balart-Sánchez, S.A. et al. Effects of Mild Traumatic Brain Injury on Resting State Brain Network Connectivity in Older Adults. Brain Imaging and Behavior 16, 1863–1872 (2022). https://doi.org/10.1007/s11682-022-00662-5
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11682-022-00662-5