Abstract
Cognitive reserve (CR) shows protective effects in Alzheimer’s disease (AD) and reduces the risk of dementia. Despite the clinical significance of CR, a clinically useful diagnostic biomarker of brain changes underlying CR in AD is not available yet. Our aim was to develop a fully-automated approach applied to fMRI to produce a biomarker associated with CR in subjects at increased risk of AD. We computed resting-state global functional connectivity (GFC), i.e. the average connectivity strength, for each voxel within the cognitive control network, which may sustain CR due to its central role in higher cognitive function. In a training sample including 43 mild cognitive impairment (MCI) subjects and 24 healthy controls (HC), we found that MCI subjects with high CR (> median of years of education, CR+) showed increased frequency of high GFC values compared to MCI-CR- and HC. A summary index capturing such a surplus frequency of high GFC was computed (called GFC reserve (GFC-R) index). GFC-R discriminated MCI-CR+ vs. MCI-CR-, with the area under the ROC = 0.84. Cross-validation in an independently recruited test sample of 23 MCI subjects showed that higher levels of the GFC-R index predicted higher years of education and an alternative questionnaire-based proxy of CR, controlled for memory performance, gray matter of the cognitive control network, white matter hyperintensities, age, and gender. In conclusion, the GFC-R index that captures GFC changes within the cognitive control network provides a biomarker candidate of functional brain changes of CR in patients at increased risk of AD.





Similar content being viewed by others
References
Achard, S., Salvador, R., Whitcher, B., Suckling, J., & Bullmore, E. (2006). A resilient, low-frequency, small-world human brain functional network with highly connected association cortical hubs. Journal of Neuroscience, 26(1), 63–72. doi:10.1523/JNEUROSCI.3874-05.2006.
Arenaza-Urquijo, E. M., Landeau, B., La Joie, R., Mevel, K., Mezenge, F., Perrotin, A., & Chetelat, G. (2013). Relationships between years of education and gray matter volume, metabolism and functional connectivity in healthy elders. NeuroImage, 83, 450–457. doi:10.1016/j.neuroimage.2013.06.053.
Ashburner, J. (2007). A fast diffeomorphic image registration algorithm. NeuroImage, 38(1), 95–113. doi:10.1016/j.neuroimage.2007.07.007.
Ashburner, J., & Friston, K. J. (2005). Unified segmentation. NeuroImage, 26(3), 839–851. doi:10.1016/j.neuroimage.2005.02.018.
Barulli, D., & Stern, Y. (2013). Efficiency, capacity, compensation, maintenance, plasticity: emerging concepts in cognitive reserve. Trends in Cognitive Sciences, 17(10), 502–509. doi:10.1016/j.tics.2013.08.012.
Bastin, C., Yakushev, I., Bahri, M. A., Fellgiebel, A., Eustache, F., Landeau, B., & Salmon, E. (2012). Cognitive reserve impacts on inter-individual variability in resting-state cerebral metabolism in normal aging. NeuroImage, 63(2), 713–722. doi:10.1016/j.neuroimage.2012.06.074.
Boots, E. A., Schultz, S. A., Almeida, R. P., Oh, J. M., Koscik, R. L., Dowling, M. N., & Okonkwo, O. C. (2015). Occupational complexity and cognitive reserve in a middle-aged cohort at risk for Alzheimer’s disease. Archives of Clinical Neuropsychology. doi:10.1093/arclin/acv041.
Bosch, B., Bartres-Faz, D., Rami, L., Arenaza-Urquijo, E. M., Fernandez-Espejo, D., Junque, C., & Molinuevo, J. L. (2010). Cognitive reserve modulates task-induced activations and deactivations in healthy elders, amnestic mild cognitive impairment and mild Alzheimer’s disease. Cortex, 46(4), 451–461. doi:10.1016/j.cortex.2009.05.006.
Bozzali, M., Dowling, C., Serra, L., Spano, B., Torso, M., Marra, C., & Cercignani, M. (2015). The impact of cognitive reserve on brain functional connectivity in Alzheimer’s disease. Journal of Alzheimer’s Disease, 44(1), 243–250. doi:10.3233/JAD-141824.
Brickman, A. M., Siedlecki, K. L., Muraskin, J., Manly, J. J., Luchsinger, J. A., Yeung, L. K., & Stern, Y. (2011). White matter hyperintensities and cognition: testing the reserve hypothesis. Neurobiology of Aging, 32(9), 1588–1598. doi:10.1016/j.neurobiolaging.2009.10.013.
Buschert, V. C., Friese, U., Teipel, S. J., Schneider, P., Merensky, W., Rujescu, D., & Buerger, K. (2011). Effects of a newly developed cognitive intervention in amnestic mild cognitive impairment and mild Alzheimer’s disease: a pilot study. Journal of Alzheimer’s Disease, 25(4), 679–694. doi:10.3233/JAD-2011-100999.
Cabeza, R., Anderson, N. D., Locantore, J. K., & McIntosh, A. R. (2002). Aging gracefully: compensatory brain activity in high-performing older adults. Neuroimage, 17(3), 1394–1402. Retrieved from http://www.ncbi.nlm.nih.gov/pubmed/12414279 http://ac.els-cdn.com/S1053811902912802/1-s2.0-S1053811902912802-main.pdf?_tid=47765b2a-94f7-11e3-b99e-00000aacb35d&acdnat=1392327732_9b8b93ea71e8f450a6a07dc8e9dace8c.
Cole, M. W., & Schneider, W. (2007). The cognitive control network: integrated cortical regions with dissociable functions. NeuroImage, 37(1), 343–360. doi:10.1016/j.neuroimage.2007.03.071.
Cole, M. W., Pathak, S., & Schneider, W. (2010). Identifying the brain’s most globally connected regions. NeuroImage, 49(4), 3132–3148. doi:10.1016/j.neuroimage.2009.11.001.
Cole, M. W., Yarkoni, T., Repovs, G., Anticevic, A., & Braver, T. S. (2012). Global connectivity of prefrontal cortex predicts cognitive control and intelligence. Journal of Neuroscience, 32(26), 8988–8999. doi:10.1523/JNEUROSCI.0536-12.2012.
Cole, M. W., Reynolds, J. R., Power, J. D., Repovs, G., Anticevic, A., & Braver, T. S. (2013). Multi-task connectivity reveals flexible hubs for adaptive task control. Nature Neuroscience, 16(9), 1348–1355. doi:10.1038/nn.3470.
Cole, M. W., Bassett, D. S., Power, J. D., Braver, T. S., & Petersen, S. E. (2014a). Intrinsic and task-evoked network architectures of the human brain. Neuron, 83(1), 238–251. doi:10.1016/j.neuron.2014.05.014.
Cole, M. W., Repovs, G., & Anticevic, A. (2014b). The frontoparietal control system: a central role in mental health. The Neuroscientist, 20(6), 652–664. doi:10.1177/1073858414525995.
Elman, J. A., Oh, H., Madison, C. M., Baker, S. L., Vogel, J. W., Marks, S. M., & Jagust, W. J. (2014). Neural compensation in older people with brain amyloid-beta deposition. Nature Neuroscience, 17(10), 1316–1318. doi:10.1038/nn.3806.
Ewers, M., Teipel, S. J., Dietrich, O., Schonberg, S. O., Jessen, F., Heun, R., & Hampel, H. (2006). Multicenter assessment of reliability of cranial MRI. Neurobiology of Aging, 27(8), 1051–1059. doi:10.1016/j.neurobiolaging.2005.05.032.
Ewers, M., Brendel, M., Rizk-Jackson, A., Rominger, A., Bartenstein, P., Schuff, N., & Alzheimer’s Disease Neuroimaging I. (2014). Reduced FDG-PET brain metabolism and executive function predict clinical progression in elderly healthy subjects. Neuroimage Clinical, 4, 45–52. doi:10.1016/j.nicl.2013.10.018.
Feis, R. A., Smith, S. M., Filippini, N., Douaud, G., Dopper, E. G., Heise, V., & Mackay, C. E. (2015). ICA-based artifact removal diminishes scan site differences in multi-center resting-state fMRI. Frontiers in Neuroscience, 9, 395. doi:10.3389/fnins.2015.00395.
Greicius, M. D., Srivastava, G., Reiss, A. L., & Menon, V. (2004). Default-mode network activity distinguishes Alzheimer’s disease from healthy aging: evidence from functional MRI. Proceedings of the National Academy of Sciences of the United States of America, 101(13), 4637–4642. doi:10.1073/pnas.0308627101.
Hall, C. B., Derby, C., LeValley, A., Katz, M. J., Verghese, J., & Lipton, R. B. (2007). Education delays accelerated decline on a memory test in persons who develop dementia. Neurology, 69(17), 1657–1664. doi:10.1212/01.wnl.0000278163.82636.30.
Jones, D. T., Machulda, M. M., Vemuri, P., McDade, E. M., Zeng, G., Senjem, M. L., & Jack, C. R., Jr. (2011). Age-related changes in the default mode network are more advanced in Alzheimer disease. Neurology, 77(16), 1524–1531. doi:10.1212/WNL.0b013e318233b33d.
Landau, S. M., Breault, C., Joshi, A. D., Pontecorvo, M., Mathis, C. A., Jagust, W. J., & Alzheimer’s Disease Neuroimaging I. (2013). Amyloid-beta imaging with Pittsburgh compound B and florbetapir: comparing radiotracers and quantification methods. Journal of Nuclear Medicine, 54(1), 70–77. doi:10.2967/jnumed.112.109009.
Li, H. J., Hou, X. H., Liu, H. H., Yue, C. L., He, Y., & Zuo, X. N. (2015). Toward systems neuroscience in mild cognitive impairment and Alzheimer’s disease: a meta-analysis of 75 fMRI studies. Human Brain Mapping, 36(3), 1217–1232. doi:10.1002/hbm.22689.
Liao, X. H., Xia, M. R., Xu, T., Dai, Z. J., Cao, X. Y., Niu, H. J., & He, Y. (2013). Functional brain hubs and their test-retest reliability: a multiband resting-state functional MRI study. NeuroImage, 83, 969–982. doi:10.1016/j.neuroimage.2013.07.058.
Luck, T., Riedel-Heller, S., & Wiese, B. (2009). CERAD-NP-Testbatterie: Alters-, geschlechts- und bildungsspezifischen Normen ausgewählter Subtests. Zeitschrift für Gerontologie und Geriatrie, 42, 372–384.
Matarazzo, J. D., & Hermann, D. O. (1984). The relationship of education and IQ in the WAIS--R standardization sample. Journal of Consulting and Clinical Psychology, 52(4), 631–634.
Members, E. C. C., Brayne, C., Ince, P. G., Keage, H. A., McKeith, I. G., Matthews, F. E., & Sulkava, R. (2010). Education, the brain and dementia: neuroprotection or compensation? Brain, 133(Pt 8), 2210–2216. doi:10.1093/brain/awq185.
Meng, X., & D’Arcy, C. (2012). Education and dementia in the context of the cognitive reserve hypothesis: a systematic review with meta-analyses and qualitative analyses. PLoS ONE, 7(6), e38268. doi:10.1371/journal.pone.0038268.
Mevel, K., Chetelat, G., Eustache, F., & Desgranges, B. (2011). The default mode network in healthy aging and Alzheimer’s disease. International Journal of Alzheimer’s Disease, 2011, 535816. doi:10.4061/2011/535816.
Nucci, M., Mapelli, D., & Mondini, S. (2012). Cognitive reserve index questionnaire (CRIq): a new instrument for measuring cognitive reserve. Aging Clinical and Experimental Research, 24(3), 218–226. doi:10.3275/7800.
Oh, H., Steffener, J., Razlighi, Q. R., Habeck, C., Liu, D., Gazes, Y., & Stern, Y. (2015). Abeta-related hyperactivation in frontoparietal control regions in cognitively normal elderly. Neurobiology of Aging, 36(12), 3247–3254. doi:10.1016/j.neurobiolaging.2015.08.016.
Otsu, N. (1979). A thresholding selection method from gray-level histogram. IEEE Transactions on Systems, Man, and Cybernetics, 9.
Petersen, R. C. (2004). Mild cognitive impairment as a diagnostic entity. Journal of Internal Medicine, 256(3), 183–194. doi:10.1111/j.1365-2796.2004.01388.x.
R Development Core Team. (2013). R: A language and environment for statistical computing. Vienna: R Foundation for Statistical Computing.
Reed, B. R., Mungas, D., Farias, S. T., Harvey, D., Beckett, L., Widaman, K., & DeCarli, C. (2010). Measuring cognitive reserve based on the decomposition of episodic memory variance. Brain, 133(Pt 8), 2196–2209. doi:10.1093/brain/awq154.
Reijnders, J., van Heugten, C., & van Boxtel, M. (2013). Cognitive interventions in healthy older adults and people with mild cognitive impairment: a systematic review. Ageing Research Reviews, 12(1), 263–275. doi:10.1016/j.arr.2012.07.003.
Rentz, D. M., Locascio, J. J., Becker, J. A., Moran, E. K., Eng, E., Buckner, R. L., & Johnson, K. A. (2010). Cognition, reserve, and amyloid deposition in normal aging. Annals of Neurology, 67(3), 353–364. doi:10.1002/ana.21904.
Sando, S. B., Melquist, S., Cannon, A., Hutton, M., Sletvold, O., Saltvedt, I., & Aasly, J. (2008). Risk-reducing effect of education in Alzheimer’s disease. International Journal of Geriatric Psychiatry, 23(11), 1156–1162. doi:10.1002/gps.2043.
Scarmeas, N., Zarahn, E., Anderson, K. E., Habeck, C. G., Hilton, J., Flynn, J., & Stern, Y. (2003). Association of life activities with cerebral blood flow in Alzheimer disease: implications for the cognitive reserve hypothesis. Arch Neurol, 60(3), 359–365. Retrieved from http://www.ncbi.nlm.nih.gov/pubmed/12633147.
Schoenberg, M. R., Dawson, K. A., Duff, K., Patton, D., Scott, J. G., & Adams, R. L. (2006). Test performance and classification statistics for the Rey auditory verbal learning test in selected clinical samples. Archives of Clinical Neuropsychology, 21(7), 693–703. doi:10.1016/j.acn.2006.06.010.
Schultz, S. A., Larson, J., Oh, J., Koscik, R., Dowling, M. N., Gallagher, C. L., & Okonkwo, O. C. (2015). Participation in cognitively-stimulating activities is associated with brain structure and cognitive function in preclinical Alzheimer’s disease. Brain Imaging and Behavior, 9(4), 729–736. doi:10.1007/s11682-014-9329-5.
Soldan, A., Pettigrew, C., Lu, Y., Wang, M. C., Selnes, O., Albert, M., & Team, B. R. (2015). Relationship of medial temporal lobe atrophy, APOE genotype, and cognitive reserve in preclinical Alzheimer’s disease. Human Brain Mapping, 36(7), 2826–2841. doi:10.1002/hbm.22810.
Solé-Padullés, C., Bartrés-Faz, D., Junqué, C., Vendrell, P., Rami, L., Clemente, I. C., & Molinuevo, J. L. (2009). Brain structure and function related to cognitive reserve variables in normal aging, mild cognitive impairment and Alzheimer’s disease. Neurobiology of Aging, 30(7), 1114–1124. Retrieved from http://www.sciencedirect.com/science/article/pii/S0197458007004083.
Stern, Y. (2002). What is cognitive reserve? Theory and research application of the reserve concept. J Int Neuropsychol Soc, 8(3), 448–460. Retrieved from http://www.ncbi.nlm.nih.gov/pubmed/11939702.
Stern, Y. (2009). Cognitive reserve. Neuropsychologia, 47(10), 2015–2028. doi:10.1016/j.neuropsychologia.2009.03.004.
Stern, Y. (2012). Cognitive reserve in ageing and Alzheimer’s disease. Lancet Neurology, 11(11), 1006–1012. doi:10.1016/s1474-4422(12)70191-6.
Stern, Y., Alexander, G. E., Prohovnik, I., & Mayeux, R. (1992). Inverse relationship between education and parietotemporal perfusion deficit in Alzheimer’s disease. Annals of Neurology, 32(3), 371–375. doi:10.1002/ana.410320311.
Stern, Y., Gurland, B., Tatemichi, T. K., Tang, M. X., Wilder, D., & Mayeux, R. (1994). Influence of education and occupation on the incidence of Alzheimer’s disease. Jama, 271(13), 1004–1010. Retrieved from http://www.ncbi.nlm.nih.gov/entrez/query.fcgi?cmd=Retrieve&db=PubMed&dopt=Citation&list_uids=8139057.
Stern, Y., Alexander, G. E., Prohovnik, I., Stricks, L., Link, B., Lennon, M. C., & Mayeux, R. (1995). Relationship between lifetime occupation and parietal flow: implications for a reserve against Alzheimer’s disease pathology. Neurology, 45(1), 55–60. Retrieved from http://www.ncbi.nlm.nih.gov/pubmed/7824135.
Stern, Y., Habeck, C., Moeller, J., Scarmeas, N., Anderson, K. E., Hilton, H. J., & van Heertum, R. (2005). Brain networks associated with cognitive reserve in healthy young and old adults. Cerebral Cortex, 15(4), 394–402. doi:10.1093/cercor/bhh142.
Stern, Y., Zarahn, E., Habeck, C., Holtzer, R., Rakitin, B. C., Kumar, A., & Brown, T. (2008). A common neural network for cognitive reserve in verbal and object working memory in young but not old. Cerebral Cortex, 18(4), 959–967. doi:10.1093/cercor/bhm134.
Suo, C., Singh, M. F., Gates, N., Wen, W., Sachdev, P., Brodaty, H., & Valenzuela, M. J. (2016). Therapeutically relevant structural and functional mechanisms triggered by physical and cognitive exercise. Molecular Psychiatry. doi:10.1038/mp.2016.19.
Valenzuela, M. J., & Sachdev, P. (2006). Brain reserve and dementia: a systematic review. Psychological Medicine, 36(4), 441–454. doi:10.1017/S0033291705006264.
Vemuri, P., Weigand, S. D., Przybelski, S. A., Knopman, D. S., Smith, G. E., Trojanowski, J. Q., & Alzheimer’s Disease Neuroimaging I. (2011). Cognitive reserve and Alzheimer’s disease biomarkers are independent determinants of cognition. Brain, 134(Pt 5), 1479–1492. doi:10.1093/brain/awr049.
Vemuri, P., Lesnick, T. G., Przybelski, S. A., Knopman, D. S., Preboske, G. M., Kantarci, K., & Jack, C. R., Jr. (2015). Vascular and amyloid pathologies are independent predictors of cognitive decline in normal elderly. Brain, 138(Pt 3), 761–771. doi:10.1093/brain/awu393.
Vossel, S., Geng, J. J., & Fink, G. R. (2014). Dorsal and ventral attention systems: distinct neural circuits but collaborative roles. The Neuroscientist, 20(2), 150–159. doi:10.1177/1073858413494269.
Wang, K., Liang, M., Wang, L., Tian, L., Zhang, X., Li, K., & Jiang, T. (2007). Altered functional connectivity in early Alzheimer’s disease: a resting-state fMRI study. Human Brain Mapping, 28(10), 967–978. doi:10.1002/hbm.20324.
Wang, J. H., Zuo, X. N., Gohel, S., Milham, M. P., Biswal, B. B., & He, Y. (2011). Graph theoretical analysis of functional brain networks: test-retest evaluation on short- and long-term resting-state functional MRI data. PLoS ONE, 6(7), e21976. doi:10.1371/journal.pone.0021976.
Wang, J., Zuo, X., Dai, Z., Xia, M., Zhao, Z., Zhao, X., & He, Y. (2013). Disrupted functional brain connectome in individuals at risk for Alzheimer’s disease. Biological Psychiatry, 73(5), 472–481. doi:10.1016/j.biopsych.2012.03.026.
Wells, R. E., Yeh, G. Y., Kerr, C. E., Wolkin, J., Davis, R. B., Tan, Y., & Kong, J. (2013). Meditation’s impact on default mode network and hippocampus in mild cognitive impairment: a pilot study. Neuroscience Letters, 556, 15–19. doi:10.1016/j.neulet.2013.10.001.
Yarkoni, T., Poldrack, R. A., Nichols, T. E., Van Essen, D. C., & Wager, T. D. (2011). Large-scale automated synthesis of human functional neuroimaging data. Nature Methods, 8(8), 665–670. doi:10.1038/nmeth.1635.
Yeo, B. T., Krienen, F. M., Sepulcre, J., Sabuncu, M. R., Lashkari, D., Hollinshead, M., & Buckner, R. L. (2011). The organization of the human cerebral cortex estimated by intrinsic functional connectivity. Journal of Neurophysiology, 106(3), 1125–1165. doi:10.1152/jn.00338.2011.
Zahodne, L. B., Manly, J. J., Brickman, A. M., Siedlecki, K. L., Decarli, C., & Stern, Y. (2013). Quantifying cognitive reserve in older adults by decomposing episodic memory variance: replication and extension. Journal of International Neuropsychological Society, 19(8), 854–862. doi:10.1017/S1355617713000738.
Zahodne, L. B., Manly, J. J., Brickman, A. M., Narkhede, A., Griffith, E. Y., Guzman, V. A., & Stern, Y. (2015). Is residual memory variance a valid method for quantifying cognitive reserve? A longitudinal application. Neuropsychologia, 77, 260–266. doi:10.1016/j.neuropsychologia.2015.09.009.
Author information
Authors and Affiliations
Consortia
Corresponding author
Ethics declarations
The study at the ISD was approved by the ethics committee of the Ludwig Maximilian University of Munich. For the ADNI-sample ethical approval was obtained by the ADNI investigators. All procedures performed were in accordance with the ethical standards of the institutional and/or national research committee and with the 1964 Helsinki declaration and its later amendments or comparable ethical standards. All study participants provided written, informed consent to the study.
Funding
The research was funded by grants of the LMUexcellent Initiative and the European Commission (ERC, PCIG12-GA-2012-334259), Alzheimer’s Forschung Initiative (AFI, DE-15035). Data collection and sharing for this project was funded by the Alzheimer’s Disease Neuroimaging Initiative (ADNI) (National Institutes of Health Grant U01 AG024904) and DOD ADNI (Department of Defense award number W81XWH-12-2-0012). ADNI is funded by the National Institute on Aging, the National Institute of Biomedical Imaging and Bioengineering, and through generous contributions from the following: AbbVie, Alzheimer’s Association; Alzheimer’s Drug Discovery Foundation; Araclon Biotech; BioClinica, Inc.; Biogen; Bristol-Myers Squibb Company; CereSpir, Inc.; Eisai Inc.; Elan Pharmaceuticals, Inc.; Eli Lilly and Company; EuroImmun; F. Hoffmann-La Roche Ltd and its affiliated company Genentech, Inc.; Fujirebio; GE Healthcare; IXICO Ltd.; Janssen Alzheimer Immunotherapy Research & Development, LLC.; Johnson & Johnson Pharmaceutical Research & Development LLC.; Lumosity; Lundbeck; Merck & Co., Inc.; Meso Scale Diagnostics, LLC.; NeuroRx Research; Neurotrack Technologies; Novartis Pharmaceuticals Corporation; Pfizer Inc.; Piramal Imaging; Servier; Takeda Pharmaceutical Company; and Transition Therapeutics. The Canadian Institutes of Health Research is providing funds to support ADNI clinical sites in Canada. Private sector contributions are facilitated by the Foundation for the National Institutes of Health (www.fnih.org). The grantee organization is the Northern California Institute for Research and Education, and the study is coordinated by the Alzheimer’s Disease Cooperative Study at the University of California, San Diego. ADNI data are disseminated by the Laboratory for Neuro Imaging at the University of Southern California.
Conflict of interest
The authors declare that they have no conflict of interest
Additional information
Data used in preparation of this article were obtained from the Alzheimer’s Disease Neuroimaging Initiative (ADNI) database (adni.loni.usc.edu). As such, the investigators within the ADNI contributed to the design and implementation of ADNI and/or provided data but did not participate in analysis or writing of this report. A complete listing of ADNI investigators can be found at: http://adni.loni.usc.edu/wp-content/uploads/how_to_apply/ADNI_Acknowledgement_List.pdf
Electronic supplementary material
Below is the link to the electronic supplementary material.
Supplementary Fig. 1
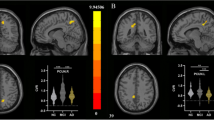
Shown is the distribution of GFC voxels averaged across subjects within CR (CR+ vs. CR-) and diagnostic (MCI vs. HC) groups for the test sample. The graphs are equivalent to Fig. 2b of the main manuscript. (GIF 14 kb)
Rights and permissions
About this article
Cite this article
Franzmeier, N., Caballero, M.Á.A., Taylor, A.N.W. et al. Resting-state global functional connectivity as a biomarker of cognitive reserve in mild cognitive impairment. Brain Imaging and Behavior 11, 368–382 (2017). https://doi.org/10.1007/s11682-016-9599-1
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11682-016-9599-1