Abstract
Programmed cell death occurs in browning explants of Fraxinus mandshurica during somatic embryogenesis, but the underlying mechanism is unclear. In this study, single cotyledons of zygotic embryos of F. mandshurica were used as explants. Mitochondrial structure and function, caspase-3-like protease activity, hydrogen peroxide metabolism, and nitric oxide accumulation induced by high concentrations of sucrose and plant growth regulators were studied. The results show that plant growth regulators induced somatic embryogenesis and also promoted explant browning. High sucrose concentrations had similar effects. High concentrations of sucrose and plant growth regulators led to the accumulation of hydrogen peroxide and nitric oxide which induced changes in mitochondrial structure and function such as modifications in mitochondrial morphology, increased membrane permeability, decreased membrane potential, and the release of cytochrome c into the cytoplasm. An increase in caspase-3-like protease activity triggered programmed cell death in some browning explant cells. During somatic embryogenesis there were increased activities of superoxide dismutase, peroxidase, and catalase, which are associated with hydrogen peroxide metabolism and jointly maintain reactive oxygen species levels. Intracellular nitric oxide synthase and nitrate reductase activities were not significantly correlated with nitric oxide content. Instead, intracellular nitric oxide may be derived from non-enzymatic reactions. Our results indicate that hydrogen peroxide and nitric oxide may function as signals, playing key roles in somatic embryogenesis and programmed cell death of explant cells of F. mandshurica. The interaction between nitric oxide and reactive oxygen species determines the occurrence of programmed cell death in explant cells; somatic embryogenesis and programmed cell death are positively regulated by hydrogen peroxide. However, the regulation of nitric oxide is complex.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Plant somatic embryogenesis (SE) is good evidence that plant cells are totipotent, capable of giving rise to any cell type. A key step in SE is the transformation of cells from somatic cells to embryonic cells (Guan et al 2016). In some plants, programmed cell death (PCD) has been observed during the formation of embryogenic cells (Petrussa et al. 2009; Rodríguez-Serrano et al. 2012; Huang et al. 2014a). However, the relationship between PCD and embryogenic cell formation, as well as its regulation mechanism, are unclear. Mitochondria regulate and amplify apoptotic signals and play a role in plant PCD processes (Zhao et al. 2018). The morphological structure of mitochondria changes when PCD occurs; mitochondria blur, their internal structure swells, and finally they rupture (Wang et al. 2009; Doorn et al. 2011). At the same time, caspase-3 activity, which is closely associated with PCD, increases (Petrussa et al. 2009). Some studies have shown that hydrogen peroxide (H2O2) and nitric oxide (NO) are involved in PCD during plant embryogenic cell formation (Huang et al. 2014a; Zhou et al. 2016). Although a relationship among PCD, NO, and H2O2 has been observed, their roles and relationships in plant embryogenic cell formation are not well understood.
Although SE has been achieved in several woody species, this form of propagation remains difficult for some mature woody plants. Fraxinus mandshurica Rupr., an important member of the genus Fraxinus, is distributed in temperate forest ecosystems in northeastern China, and has a high timber value (Gu et al. 2015). Technology for SE in this species has been studied for 15 years, and a direct SE system using zygotic cotyledons as explants has been established. Seedlings regenerated using this technology have been planted in the field and continue to grow well (Yang et al. 2013). In the process of embryogenic cell production in F. mandshurica, however, some explants show browning, which can lead to the death of the cells and limit the production of regenerated plants. In addition, the browning of explants seems likely to be related to programmed cell death because the levels of intracellular reactive oxygen species (ROS) increase during the browning process (Liu et al. 2015; Yang et al. 2019). There is a need to explore the roles of signalling molecules such as ROS in the transformation of somatic cells into embryogenic cells, and to clarify their relationships with PCD.
In this study, changes were monitored in mitochondrial structure and function, caspase-3 enzyme activity, intracellular H2O2 metabolism, and NO accumulation during SE in explants under high concentrations of sucrose and in response to plant growth regulators (PGRs). The objectives were to explore: (1) the functions of high concentrations of sucrose in SE and PCD of explant cells; and, (2) the functions of, and relationships between, H2O2 and NO in SE and PCD of explant cells. Our results should provide insights into the roles of signalling molecules (e.g., ROS) in the process of the transformation of somatic cells into embryogenic cells and their relationship with PCD, and may provide experimental evidence for the regulation of SE and PCD in F. mandshurica.
Materials and methods
Experimental materials
Mature dehydrated seeds (Fig. 1a) were collected in late September 2015 from a mature mother tree of F. mandshurica growing at the Shanhetun Forestry Bureau, Wuchang City, Heilongjiang Province. Single cotyledons of zygotic embryos were used as explants.
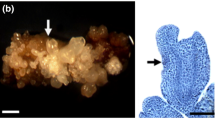
Explant browning and somatic embryogenesis of Fraxinus mandshurica at day 60 of culture, a mature samara (1), endosperm-coated embryos (2) and cotyledon-type embryos filled with embryonic cavities (3); b–d single cotyledon cultured on medium with 75 g·L−1 sucrose + 26.84 μM NAA + 8.88 μM BA. b explant browning. c somatic embryos in different developmental stages. d “Y” shaped vascular tissue of somatic embryo; e and f single cotyledon cultured on medium with 20 g·L−1 sucrose + 26.84 μM NAA + 8.88 μM BA. e spherical embryos; f spherical somatic embryo with radiation symmetry structure. g single cotyledon cultured on medium with 75 g·L−1 sucrose. The explant turned brown, but there were no somatic embryos. h single cotyledon cultured on medium with 20 g·L−1 sucrose. The explant did not turn brown, and no somatic embryos were observed. coty: cotyledons; se: somatic embryo; pvb: primary vascular bundle; bar = 1 cm (a and b), 5 mm (c, e, g, h), 1.25 mm (d), 1 mm (f)
Methods
Effect of high sucrose and PGRs concentrations on somatic embryogenesis
Material pretreatment
Based on Yang et al. (2013), the collected seeds were mixed evenly and their pericarps removed. The seeds were soaked in running tap water for two days, then agitated in 70% (v/v) aqueous ethanol for 1 min, surface sterilized in 3% (v/v) aqueous sodium hypochlorite for 15 min, and finally rinsed five times with sterile distilled water. Single cotyledons from zygotic embryos were inoculated onto the surface of the medium, with the inner side of the cotyledon in contact with the medium.
Somatic embryo induction and extraction methods
The methods for SE induction and extraction were those described by Yang et al. (2013), with some modifications. The basic medium for SE induction was ½-strength Murashige and Skoog medium (½-MS), with the addition of 26.84 μM naphthalene acetic acid (NAA) and 8.88 μM benzyl adenine (BA) or without PGRs, 400 mg·L−1 casein hydrolysate (CH), 75 or 20 g·L−1 sucrose, and 6.5 g·L−1 agar (Beijing Aoboxing Biotechnology Co., Ltd., Beijing, China). The pH of all media was adjusted to 5.8 before autoclaving. The explants were inoculated onto the different media and cultured for 60 d in the dark at 23–25 °C. Each treatment consisted of 10 petri dishes, each inoculated with 10 explants, and each treatment repeated three times. On day 60, the occurrence of somatic embryos was recorded, and samples were collected for paraffin sectioning (Yang et al. 2019).
Cultured materials collected at the beginning and on day 13 were observed by transmission electron microscopy. Mitochondrial function indexes, caspase-like enzyme and antioxidant enzyme activities, intracellular H2O2 and NO contents, and the activities of NO synthesis-related enzymes were determined on days 5, 7, 9, 11 and 13. Samples were randomly selected for the measurements of each parameter.
Effects of high sucrose and PGRs concentrations on PCD
Sample preparation for transmission electron microscope observations
Cultured materials were collected at different times, cut into 3 mm long × 1 mm wide pieces, immediately placed in 2.5% (v/v) glutaraldehyde solution and kept at 0–4 °C in the dark for 12–48 h. The samples were then removed and washed three times with 0.1 mmol·L−1 phosphate buffer (pH 6.8) for 15 min each time, followed by the addition of 1% (v/v) osmic acid solution and further incubation for 4 h. Samples were finally rinsed three times with 0.1 mmol·L−1 phosphate buffer (pH 6.8) for 15 min each time. The fixed samples were dehydrated in successive steps by immersion in different concentrations of ethanol: 50% (v/v) → 70% (v/v) → 90% (v/v) → 100% (v/v) ethanol, for 20 min in each followed by immersion in a 1 : 1 (v/v) mixture of 100% ethanol and 100% acetone for 20 min, and finally in 100% acetone for 10 min. The dehydration temperature was 0–4 °C. For immersion and embedding, pure acetone was mixed with an embedding agent (domestic epoxy resin 618) = 1 : 1 (v/v), 1 : 2 (v/v) and 1 : 3 (v/v)) as a penetrant for soaking. For each ratio, the permeation treatment time was 1, 5, and 24 h, respectively, at room temperature. Samples were immediately placed in a 1.5 mL centrifuge tube, and penetrant was added. The embedding agent was injected into the hole of the embedding block and the sample removed from the centrifuge tube and placed in the hole of the embedding block. The sample was polymerized in an incubator at 60 °C for 48 h, removed, and the block and light mirror positioning were adjusted. Sectioning was carried out with a LKB-5 microtome, to an approximate thickness of 50–70 nm. The sample was subsequently double-dyed with lead citrate and 2% uranyl acetate, and observed and photographed under a Tianmei Hitachi H-7650 electron microscope (Hitachi Ltd., Tokyo, Japan).
Analysis of mitochondrial function indicators
Mitochondria were extracted according to Huang et al. (2014b). In a pre-cooled mortar, 0.3 g samples were ground with 2 mL extraction buffer [0.05 mol L−1 tris–HCl buffer (pH 7.4), 1 mmol L−1 ethylenediaminetetraacetic acid disodium salt (EDTA-Na), 0.4 mol L−1 sucrose] into a homogenate. This was centrifuged at 1,800 g for 15 min at 4 °C. The supernatant was collected and then centrifuged at 5,400 g for 15 min at 4 °C. The precipitate was washed three times with extraction buffer and suspended in the same buffer but without EDTA-Na. The mitochondrial concentration was expressed as mitochondrial protein content (mg), and protein content was determined by the Coomassie brilliant blue method. Mitochondrial membrane permeability analysis was carried out according to Huang et al. (2014b), and mitochondrial membrane potential and Cytochrome c/a were determined following Braidot et al. (1998) and Tonshin et al. (2003), respectively.
Intracellular caspase-3-like protease assay
The assay was carried out according to the KGI caspase-3-like protease activity spectrophotometric detection kit (KGA203F, Jiangsu Kaiji Biotechnology Co., Ltd., Jiangsu, China). The fresh material was washed twice with phosphate-buffered saline and placed in a mortar in an ice bath. Subsequently, 150–200 μL pre-cooled lysis buffer with 0.5 μL DTT per 50 μL lysis buffer added before use, and the mixture was vortexed twice for 5 min each time. The supernatant, containing the lysed protein, was transferred into a new pre-cooled centrifuge tube and placed on ice. A small amount of supernatant (1–2 μL) was collected and the protein concentration was determined according to Bradford (1976). For each sample, 50 μL supernatant containing 100–200 μg protein was collected. If the volume was less than 50 μL, 10 mmol·L−1 PBS (pH 7.2–7.6) was added to obtain a total volume of 50 μL (all groups were measured and compared based on the same amount of protein). Subsequently, 50 μL 2 × reaction buffer, with 0.5 μL DTT added per 50 μL 2 × reaction buffer before use, and 5 μL caspase-3-like protease active substrate were added. The mixture was incubated for 4 h at 37 °C in the dark. Absorbance was measured with a spectrophotometer (Ultrospec 2100 pro, Amersham Pharmacia Biotech, Little Chalfont, UK) at λ = 405 nm. Caspase-3-like protease activity was determined as the multiple in the optical density (OD) of apoptotic cells over that of the negative control. The blank control contained a mixture of 50 μL lysis buffer and 50 μL reaction buffer.
Effect of high concentration of sucrose and PGRs on H2O2 metabolism
Intracellular H2O2 levels were determined according to Zhan et al. (2014). The 0.5 g samples were homogenized in an ice bath with 2 mL 0.1% (w/v) cold trichloroacetic acid (TCA). The homogenate was centrifuged at 15,000 g for 15 min at 4 °C and 0.5 mL of the supernatant was added to 0.5 mL of 10 mmol·L−1 potassium phosphate buffer (pH 7.0) and freshly prepared 1 mL of 1 mol·L−1 potassium iodide (KI) was added to 10 mmol L−1 potassium phosphate buffer (pH 7.0). The reaction mixture was then analyzed for absorbance at λ = 390 nm, and H2O2 concentration was obtained using standard curve. The results are expressed as μmol·g−1 Pro.
For the assessment of antioxidant enzymes, 0.25 g fresh samples were crushed with 50 mmol·L−1 phosphate buffer (pH 7.8). The homogenate was centrifuged at 12,000 g for 20 min at 4 °C to produce the supernatant. Superoxide dismutase (SOD), peroxidase (POD), and catalase (CAT) activities were based on Zhan et al. (2014).
SOD activity was determined by nitro blue tetrazolium chloride (NBT) photoreduction. A 50 μL supernatant was added to the reaction solution containing 1.5 mL of 50 mmol L−1 potassium phosphate buffer (pH 7.8), 0.3 mL 130 mmol L−1 methionine, 0.3 mL 750 μmol L−1 NBT, 0.3 mL 100 μmol L−1 EDTA-Na2, 0.3 mL 20 μmol·L−1 riboflavin, and 0.3 mL sterile deionized water. The mixture was positioned 30 cm away from an iodine lamp with 800 μmol·m−2·s−1 for 15 min at 25 °C. The reaction mixture was then analyzed for absorbance at 560 nm. One unit of SOD is defined by the amount of enzyme that inhibits NBT photo-reduction by 50%. SOD activity was expressed as unit·g−1 Pro.
POD activity was detected by the guaiacol oxidation method. A 50 μL supernatant was added to a reaction solution containing 1.0 mL 30% H2O2 (v/v), 1.0 mL of 0.2 mol L−1 potassium phosphate buffer (pH 6.0), and 0.95 mL 0.2% guaiacol. The mixture was then analyzed for absorbance at 470 nm. One unit of POD was defined as an OD of 0.01. The specific activity of POD was given as unit·g−1 Pro·min−1.
CAT activity was assayed by the decomposition of H2O2. A 100 μL supernatant was mixed with a reaction solution containing 1.9 mL of 0.1 mol·L−1 potassium phosphate buffer (pH 7.0) and 1 mL 0.2% H2O2 (v/v). The decrease in H2O2 was monitored at 240 nm and quantified by its molar extinction coefficient. CAT activity was expressed as mg H2O2·g−1 Pro·min−1.
Effect of high concentration of sucrose and PGRs on NO synthesis
Nitric oxide content was determined according to Libourel et al. (2006). Half gram samples were homogenized in an ice bath with 2 mL cold sterile deionized water (pH 8.0). A 50.0 μL of 0.5 mol·L−1 sodium hydroxide (NaOH) and 10.0 μL of 0.4 mol·L−1 zinc sulfate (ZnSO4) were added to the homogenate. The mixture was placed in 60 °C water for 10 min. The mixture was then centrifuged at 5,400 g for 15 min at 4 °C and 1.0 mL of the supernatant was added to a 1.0 mL reaction solution containing 1% sulfanilamide (w/v, in 5% phosphoric acid) and 0.1% N-1-naphthyl-aminophylline flurazepam (w/v, in sterile deionized water). The mixture was placed in the dark for 15 min at 25 °C, after which it was analyzed for absorbance at 540 nm. The concentration of sodium nitrite (NaNO2) was obtained using standard curves, and the results expressed as μmol·g−1 Pro.
Nitric oxide synthase (NOS) activity was assayed with an NOS assay kit (NOS A014-2, Nanjing Jiancheng Biotechnology Institute, Nanjing, China). For enzyme extraction, 0.25 g samples were placed in a pre-cooled mortar (30 min at − 20 °C), 4 mL of a 0.1 mol·L−1 phosphate buffer (pH 7.4) were added, and the mixture was ground on ice into a homogenate. This was centrifuged at 7000 g for 15 min and served as the enzyme solution. For NOS activity, the absorbance was determined at 530 nm (light path, 1 cm) against double distilled water as the blank. The activity of NOS was calculated from the absorbance value.
Nitrate reductase (NR) activity was determined with an NR kit (NR A096, Nanjing Jiancheng Bioengineering Institute, Nanjing, China). For enzyme extraction, each sample was mechanically homogenized with nine times the volume of the buffer (1 : 9, w/v) in an ice-water bath. The homogenate was centrifuged at 6000 g for 10 min and used as the enzyme extract. For NR activity, absorbance was determined at 540 nm (light path, 1 cm) against double distilled water as the blank. The OD of each sample was used to calculate NR activity.
Data analysis and statistics
Each treatment was repeated three times. All indexes were calculated using Microsoft Excel 2007 and differences were analysed by one-way ANOVA and Duncan’s multiple comparison (Duncan, α = 0.05) using SPSS software (v21.0, SPSS Inc.). Pearson’s method of skewness was used for correlation analysis. Graphs were plotted using Sigmaplot software (v12.5, SYSTAT). Indexes of SE were calculated as follows:
Results
Effect of high concentration of sucrose and PGRs on browning and somatic embryogenesis
The sucrose and PGRs treatments affected explant browning and somatic embryogenesis (Fig. 1b–h). Higher concentrations of sucrose promoted explant browning. The percentage of browning explants differed significantly among the treatments by day 60 (Fig. 2a). The percentage on medium containing 75 g·L−1 sucrose and no PGRs was 94.2%, 112.2% higher than on medium containing 20 g·L−1 sucrose and no PGRs. The presence of PGRs increased the percentage of browning explants. The percentage of browning explants was 100% on medium containing 75 g·L−1 sucrose + PGRs, and 46.1% on medium containing 20 g·L−1 sucrose + PGRs.
No somatic embryos formed from explants on media without PGRs. In the medium containing 75 g·L−1 sucrose + PGRs (Fig. 2b), SE was 58.6%, more than five times higher than on medium containing 20 g·L−1 sucrose + PGRs (11.4%). The number of somatic embryos was higher in explants on medium containing 75 g·L−1 sucrose (20.0 somatic embryos per explant) than in explants on medium containing 20 g·L−1 sucrose (14.6 somatic embryos per explant) (Fig. 2c).
Effect of high concentrations of sucrose and PGRs on programmed cell death
The effect of sucrose on mitochondrial structure was not influenced by PGRs in the medium (Fig. 3). Before culturing, cotyledon cells covered with fat granules were arranged in a regular pattern (Fig. 3a). However, after 13 days of culturing chromatin condensation had occurred inside the nuclei of explant cells on media containing 75 g·L−1 sucrose (with or without PGRs). The mitochondria had a blurred structure and spherical shape (Fig. 3b and d). In contrast, the morphology and structure of mitochondria were normal in explant cells on media containing 20 g·L−1 sucrose (with or without PGRs) (Fig. 3c and e).
Changes in mitochondria and nuclei in cells of Fraxinus mandshurica by day 13 of culture. a Explant cells before culture; b Explants in medium with 75 g·L−1 sucrose + 26.84 μM NAA + 8.88 μM BA after 13 days in culture. Chromatin condensates inside the nucleus and the internal structure of mitochondria become blurred and shows a spherical shape; c Explants in medium with 20 g·L−1 sucrose + 26.84 μM NAA + 8.88 μM BA after 13 days in culture; d Explants on medium with 75 g·L−1 sucrose after 13 days in culture; e Explants on medium with 20 g·L−1 sucrose after 13 days in culture. Mitochondrial morphology is normal and nuclear chromatin is uniform. Bar = 2 μm (a–e). M Mitochondrion; N Nucleus
Sucrose and the presence of PGRs in the culture medium significantly affected mitochondrial function (Fig. 4) and intracellular caspase-3-like protease activity (Fig. 5). As the culturing increased, mitochondrial membrane absorbance (Fig. 4a), membrane potential (Fig. 4b), and mitochondrial Cyt c/a (Fig. 4c) decreased (P9d < 0.05, P11d−13 d < 0.01), while caspase-3-like protease activity increased (P5d−11d < 0.05, P13d < 0.01, Fig. 5). When explants were on the high sucrose concentration medium, mitochondrial membrane potential and absorbance decreased, while caspase-3-like protease activity increased during the culturing period. For a given sucrose concentration, mitochondrial membrane absorbance and potential were lower, and caspase-3-like protease activity higher if PGRs were present.
Effect of high concentrations of sucrose and PGRs on H2O2 metabolism
High concentrations of sucrose and PGRs increased H2O2 level in explants. As culturing time extended, intracellular H2O2 levels increased in all treatments (P5d−13 d < 0.01, Fig. 6a). At various times, the lowest intracellular H2O2 level was in explants on medium containing 20 g·L−1 sucrose and no PGRs, while the highest was in explants on medium containing 75 g·L−1 sucrose + PGRs.
High concentrations of sucrose increased SOD activity while PGRs had the opposite effect. The highest SOD activity was in explants on medium containing 75 g·L−1 sucrose and no PGRs, except on day 11. During the culturing period, SOD activity first increased and then decreased in all treatments (P5d > 0.05, P7d−11 d < 0.05, P13d < 0.01, Fig. 6b), whereas POD activity increased continuously until the end of the culturing period (P5d > 0.05, P7d−13 d < 0.01, Fig. 6c). For explants on medium containing 20 g·L−1 sucrose and no PGRs, SOD activity increase was delayed, reaching a maximum on day 11 (compared with day 9 in the other three treatments) (Fig. 6b).
Both concentrations of sucrose and PGRs promoted intracellular POD activity, which reached a maximum level (0.832 unit·g−1 Pro·min−1) on medium containing 75 g·L−1 sucrose + PGRs by day 13 (Fig. 6c). This activity was markedly higher than in the other treatments; the lowest POD activity (0.366 unit·g−1 Pro min−1) was in explants on medium containing 20 g·L−1 sucrose and no PGRs.
Both concentrations of sucrose and PGRs inhibited intracellular CAT activity in explants. As culturing time extended, CAT activity first increased, then decreased, and then increased again, but the timing of the fluctuations varied among the treatments (Fig. 6d). The first peak in CAT activity was on day 9 in explants on medium containing 75 g·L−1 sucrose + PGRs, two days later than in the other treatments. On day 13, CAT activity increased to 1.39 mg H2O2·g−1 Pro·min−1 in explants on medium containing 20 g·L−1 sucrose and no PGRs. This was significantly higher than in the other treatments and 105.7% higher than the lowest CAT activity (0.68 mg H2O2· g−1 Pro·min−1) in explants on medium containing 75 g·L−1 sucrose + PGRs.
Effect of high concentrations of sucrose and PGRs on enzymes related to NO synthesis
Effects of sucrose levels and PGRs on intracellular NO synthesis did not show a distinct regular pattern. As the culture time extended, nitric oxide first increased and then decreased in all treatments, but the timing of peak NO levels differed among treatments (Fig. 7a).
High concentrations of sucrose and PGRs inhibited NOS. As culturing time extended, NOS activity increased and then decreased in all treatments. NOS activity was significantly higher in explants on medium with 20 g·L−1 sucrose and no PGRs than in explants on the other media (Fig. 7b). The highest NOS activity (4.4 unit·mg−1 Pro) was on day 13 of culturing on medium containing 20 g·L−1 sucrose and no PGRs. This was 3.4 times higher than that in explants on medium with 75 g·L−1 sucrose + PGRs (1.30 unit·mg−1 Pro).
Sucrose concentrations and PGRs also affected NR activity. As culturing time increased, NR activity increased in explants on medium containing 20 g·L−1 sucrose and no PGRs. NR activity increased and then decreased in the other three treatments but the time of peak activity differed among the treatments (Fig. 7c). The peak in NR was on day 7 in explants on medium containing 75 g·L−1 sucrose and no PGRs, on day 9 on medium containing 75 g·L−1 sucrose + PGRs, on medium containing 20 g·L−1 sucrose + PGRs, and on day 13 on medium containing 20 g·L−1 sucrose and no PGRs (Fig. 7c).
Effect of high concentrations of sucrose and PGRs on NO/H2O2 ratios
The ratio of NO to H2O2 was reduced by high sucrose levels and PGR treatments and was consistently higher in explants treated with 20 g·L−1 sucrose and no PGRs than in the other treatments. As the culturing time extended, NO/H2O2 ratios decreased in all treatments (P5d < 0.05, P7 d−13 d < 0.01, Fig. 8). By day 5, the highest NO/H2O2 ratio (230.1) was in explants on medium containing 20 g·L−1 sucrose and no PGRs, and was significantly higher than those in other treatments. The lowest NO/H2O2 ratio (19.6) was in explants on medium containing 75 g·L−1 sucrose and no PGRs.
Discussion
High sucrose concentrations promote somatic embryogenesis induced by plant growth regulators
Plant growth regulators are necessary for SE in species such as F. mandshurica and F. excelsior (Capuana et al. 2007). Our results also show that high sucrose concentrations can promote SE in explants of F. mandshurica. This is consistent with the results of Eapen and George (1993) for peanut (Arachis hypogaea L.). Similarly, Krishnan and Siril (2017) suggested that prolonged nutritional stress along with high levels of exogenous auxins might lead to a higher percentage of embryo induction from callus tissues. The presence of auxin in the medium may affect gene expression in differentiating cells of Arabidopsis by increasing demethylation of DNA (Grzybkowska et al. 2018). They reported a decrease in the global DNA methylation levels during SE that contrasted with the up-regulation of METHYLTRANSFERASE 1 (MET1) and CHROMOMETHYLASE 3 (CMT3) DNA methylases and the down-regulation of DNA demethylases, including REPRESSOR OF SILENCING 1 (ROS1), DEMETER (DME), and DEMETER-LIKE 2 (DML2).
High sucrose and PGRs concentrations promote programmed cell death during somatic embryogenesis
Yang et al. (2019) detected DNA fragmentation in the nucleus of explant cells during the induction of SE in F. mandshurica, suggesting that somatic embryogenesis in this species is accompanied by PCD of some explant cells. Mitochondria are important organelles in plant cells. They are regulators and amplifiers of apoptotic signals and are involved in PCD (Zhao et al. 2018). Damage to mitochondrial structure often occurs in the late stages of apoptosis or cell death, and is a typical marker of PCD (Doorn et al. 2011). In this study, when cotyledons of F. mandshurica were cultured on medium containing 75 g·L−1 sucrose for 13 days, the internal structure of the mitochondria became blurred (Fig. 3), indicative of functional damage. These results provide evidence that high concentration of sucrose causes programmed cell death in some explant cells during somatic embryogenesis of F. mandshurica.
Changes in mitochondrial membrane absorbance are indicative of changes in permeability (Zhan et al. 2014). In this study, the higher the sucrose concentration in the medium, the higher the mitochondrial membrane permeability. This supports the finding that PCD induced by high sucrose and PGR concentrations is dependent on mitochondrial membrane pore openings, which affects permeability (Panda et al. 2008). Membrane permeability increased and membrane potential decreased; at the same time, mitochondrial Cyt c decreased during somatic embryogenesis (this study; Zhan et al. 2014). The higher the concentration of sucrose in the medium, the lower the Cyt c/a ratio, and the presence of PGRs increased the magnitude of this reduction. Thus, the results show that mitochondrial membrane structure and function are seriously damaged during PCD induced by high concentration of sucrose and PGRs.
The results also show that caspase-like protease is involved in the process of programmed cell death in somatic embryogenesis of F. mandshurica. In this study, the highest caspase-3-like protease activity was in explants on medium containing 75 g·L−1 sucrose + PGRs (NAA + BA), and the activation of enzyme activity was also faster in this treatment than in the other treatments (Fig. 5). This is consistent with the results of Biswas and Mano (2016) who reported that lipid peroxide-derived reactive carbonyl species (RCS), which are downstream products of ROS, mediate oxidative signals to initiate PCD in plants.
H2O2 in the induction of PCD during somatic embryogenesis
The results show that H2O2 plays an important role in somatic embryogenesis of F. mandshurica. As culturing time extended, the H2O2 levels increased during SE. At a given culture point, the H2O2 was related to the rate of SE, i.e., highest in the 75 g·L−1 sucrose + PGRs treatment, followed by the 20 g·L−1 sucrose + PGRs treatment, the 75 g·L−1 sucrose and no PGRs treatment, and lowest in the 20 g·L−1 sucrose and no PGRs treatment (Figs. 2b and c, 6a). Similar results have been reported for Gossypium hirsutum L. (Cheng et al. 2015) and Larix leptolepis (Lamb.) Carr. (Zhou et al. 2016), suggesting that higher H2O2 induced by higher sucrose and PGRs concentrations may be a key condition for SE in plants. In addition, in the correlation analyses, H2O2 levels were significantly associated with SOD, POD, and CAT activity (Supplementary Tables S1, S2, S3, and S4). Its metabolism was closely related to SE in F. mandshurica. This result is consistent with observations in other woody species as reported by Yang et al. (2019) and Zhang et al. (2010). Salo et al. (2016) found that entry into the embryogenic pathway is related to cellular stress responses during somatic embryogenesis in Scots pine (Pinus sylvestris L.). Their results indicated that high sucrose concentration triggers the embryogenic pathway and activates a wide range of stress defence mechanisms. Thus, the manipulation of stress response pathways may provide a way to enhance SE in recalcitrant F. mandshurica lines.
Crosstalk between H2O2 and NO in the SE and PCD of explant cells
As a secondary messenger in plants, NO plays an important role in biological activities. Recent studies have explored the crosstalk between H2O2 and NO signals (Ahmad et al. 2019). In this study, correlation analysis showed that NO levels in explants on medium containing 75 g·L−1 sucrose + PGRs were significantly correlated with the activities of antioxidant enzymes (SOD, POD, and CAT) (Table S1) but not significantly correlated with H2O2. This suggests that intracellular NO levels affect H2O2 metabolism, and the NO signal is located upstream of the H2O2 signal. This is similar to the results of Yao et al. (2012) but in contrast to Lum et al. (2002). In addition, Huang et al. (2014a) showed that the NO signal is located upstream of the H2O2 signal in the signalling network regulating haemoglobin-controlled cell survival or death during in vitro plant embryogenesis.
Both NOS and NR are potential sources of intracellular NO (Deng et al. 2016). The activities of these two enzymes were monitored during somatic embryogenesis and there were no significant correlations between intracellular NO content and NOS or NR activities (Tables S1, S2, S3 and S4). Instead, there may be non-enzymatic reactions related to NO synthesis during the SE process (Ahmad et al. 2019).
The interaction between NO and ROS determines the occurrence of PCD (Balestrazzi et al. 2011; Wilkins et al. 2011). In this study, caspase-3-like protease activity showed a positive correlation with intracellular H2O2 in treatments with 75 g·L−1 sucrose + PGRs and with 20 g·L−1 sucrose + PGRs. Intracellular NO and NO/H2O2 ratios were significantly negative with caspase-3-like protease activity (Tables S1 and S2). However, NO content in explants on a medium of 75 g·L−1 sucrose + PGRs was significantly higher than on 20 g·L−1 sucrose + PGRs at 5, 9 and 11 days, and explant browning was significantly higher with 75 g·L−1 sucrose + PGRs than with 20 g·L−1 sucrose + PGRs, as well as caspase-3-like protease activity. These results indicate that H2O2 is a positive regulator of somatic embryogenesis in F. mandshurica and programmed cell death of explant cells, while the regulation of nitric oxide is complex.
In summary, plant growth regulators play a decisive role in somatic embryogenesis of F. mandshurica, and promoted by H2O2 and NO signals caused by high sucrose concentration. In addition, H2O2 is involved in the induction of programmed cell death in explant cells, and is related to NO synthesis. Our findings imply that H2O2 is a positive regulator of somatic embryogenesis in F. mandshurica and programmed cell death while NO regulation is complex. The manipulation of stress response pathways may provide a way to enhance somatic embryogenesis in recalcitrant F. mandshurica lines.
Change history
23 February 2022
A Correction to this paper has been published: https://doi.org/10.1007/s11676-021-01398-6
References
Ahmad P, Tripathi DK, Deshmukh R, Singh VP, Corpas FJ (2019) Revisiting the role of ROS and RNS in plants under changing environment. Environ Exp Bot 161:1–3
Balestrazzi A, Agoni V, Tava A, Biazzi E, Raimondi E, Macovei A, Carbonera D (2011) Cell death induction and nitric oxide biosynthesis in white poplar (Populus alba) suspension cultures exposed to alfalfa saponins. Physiol Plant 141(3):227–238
Biswas MS, Mano J (2016) Reactive carbonyl species activate caspase-3-like protease to initiate programmed cell death in plants. Plant Cell Physiol 57(7):1432–1442
Bradford MM (1976) A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal Biochem 72(1–2):248–254
Braidot E, Petrussa E, Macrì F, Vianello A (1998) Plant mitochondrial electrical potential monitored by fluorescence quenching of rhodamine 123. Biol Plantarum 41(2):193–201
Capuana M, Petrini GMA, Giannini R (2007) Plant regeneration of common ash (Fraxinus excelsior L.) by somatic embryogenesis. Vitro Cell Dev-Pl 43(2):101–110
Cheng WH, Wang FL, Cheng XQ, Zhu QH, Sun YQ, Zhu HG, Sun J (2015) Polyamine and its metabolite H2O2 play a key role in the conversion of embryogenic callus into somatic embryos in upland cotton (Gossypium hirsutum L.). Front Plant Sci 6(319):1063
Deng XG, Zhu T, Zou LJ, Han XY, Zhou X, Xi DH, Zhang DW, Lin HH (2016) Orchestration of hydrogen peroxide and nitric oxide in brassinosteroids mediated systemic virus resistance in Nicotiana benthamiana. Plant J 85(4):478–493
Doorn W, Beers EP, Dangl JL, Franklin-Tong VE, Gallois P, Hara-Nishimura I, Jones AM, Kawai-Yamada M, Lam E, Mundy J, Mur LAJ, Petersen M, Smertenko A, Taliansky M, Van Breusegem F, Wolpert T, Woltering E, Zhivotovsky B, Bozhkov PV (2011) Morphological classification of plant cell deaths. Cell Death Differ 18(8):1241–1246
Eapen S, George L (1993) Somatic embryogenesis in peanut: influence of growth regulators and sugars. Plant Cell Tiss Org 35(2):151–156
Grzybkowska D, Morończyk J, Wójcikowska B, Gaj MD (2018) Azacitidine (5-AzaC)-treatment and mutations in DNA methylase genes affect embryogenic response and expression of the genes that are involved in somatic embryogenesis in Arabidopsis. Plant Growth Regul 85:243–256
Gu JC, Wei X, Wang J, Dong XY, Wang ZQ (2015) Marked differences in standing biomass, length density, anatomy and physiological activity between white and brown roots in Fraxinus mandshurica Rupr plantation. Plant Soil 392(1–2):267–277
Guan Y, Li SG, Fan XF, Su ZH (2016) Application of somatic embryogenesis in woody plants. Front Plant Sci 7:938
Huang SL, Hill RD, Wally OSD, Dionisio G, Ayele BT, Jami SK, Stasolla C (2014a) Hemoglobin control of cell survival/death decision regulates in vitro plant embryogenesis. Plant Physiol 165(2):810–825
Huang WJ, Oo TL, He HY, Wang AQ, Zhan J, Li CZ, Wei SQ, He LF (2014b) Aluminum induces rapidly mitochondria-dependent programmed cell death in Al-sensitive peanut root tips. Bot Stud 55(1):67
Krishnan SRS, Siril EA (2017) Auxin and nutritional stress coupled somatic embryogenesis in Oldenlandia umbellata L. Physiol Mol Biol Plants 23:471–475
Libourel IGL, Bethke PC, Michele RD, Jones RL (2006) Nitric oxide gas stimulates germination of dormant Arabidopsis seeds: use of a flow-through apparatus for delivery of nitric oxide. Planta 223(4):813–820
Liu CP, Yang L, Shen HL (2015) Proteomic analysis of immature Fraxinus mandshurica cotyledon tissues during somatic embryogenesis: effects of explant browning on somatic embryogenesis. Int J Mol Sci 16(6):13692–13713
Lum HK, Butt YK, Lo SC (2002) Hydrogen peroxide induces a rapid production of nitric oxide in mung bean (Phaseolus aureus). Nitric Oxide-Biol Ch 6(2):205–213
Panda SK, Yamamoto Y, Kondo H, Matsumoto H (2008) Mitochondrial alterations related to programmed cell death in tobacco cells under aluminum stress. C R Biol 331(8):597–610
Petrussa E, Bertolini A, Casolo V, Krajnáková J, Macrì F, Vianello A (2009) Mitochondrial bioenergetics linked to the manifestation of programmed cell death during somatic embryogenesis of Abies alba. Planta 231(1):93–107
Rodríguez-Serrano M, Bárány I, Prem D, Coronado MJ, Risueño MC, Testillano PS (2012) NO, ROS, and cell death associated with caspase-like activity increase in stress-induced microspore embryogenesis of barley. J Exp Bot 63(5):2007–2024
Salo HM, Sarjala T, Jokela A, Häggman H, Vuosku J (2016) Moderate stress responses and specific changes in polyamine metabolism characterize Scots pine somatic embryogenesis. Tree Physiol 36:392–402
Tonshin AA, Saprunova VB, Solodovnikova IM, Bakeeva LE, Yaguzhinsky LS (2003) Functional activity and ultrastructure of mitochondria isolated from myocardial apoptotic tissue. Biochemistry (mosc) 68(8):875–881
Wang L, Li X, Chen S, Liu G (2009) Enhanced drought tolerance in transgenic Leymus chinensis plants with constitutively expressed wheat talea3. Biotechnol Lett 31(2):313–319
Wilkins KA, Bancroft J, Bosch M, Ings J, Smirnoff N, Franklin-Tong VE (2011) Reactive oxygen species and nitric oxide mediate actin reorganization and programmed cell death in the self-incompatibility response of papaver. Plant Physiol 156(1):404–416
Yang L, Bian L, Shen HL, Li YH (2013) Somatic embryogenesis and plantlet regeneration from mature zygotic embryos of Manchurian ash (Fraxinus mandshurica Rupr). Plant Cell Tiss Org 15(2):115–125
Yang L, Wei C, Huang C, Liu HN, Zhang DY, Shen HL, Li YH (2019) Role of hydrogen peroxide in stress-induced programmed cell death during somatic embryogenesis in Fraxinus mandshurica. J Forestry Res 30(3):767–777
Yao LL, Pei BL, Zhou Q, Li YZ (2012) NO serves as a signaling intermediate downstream of H2O2 to modulate dynamic microtubule cytoskeleton during responses to vd-toxins in Arabidopsis. Plant Signal Behav 7(2):174–177
Zhan J, Li W, He HY, Li CZ, He LF (2014) Mitochondrial alterations during Al-induced PCD in peanut root tips. Plant Physiol Biochem 75:105–113
Zhang SG, Han SY, Yang WH, Wei HL, Ming Z, Qi LW (2010) Changes in H2O2 content and antioxidant enzyme gene expression during the somatic embryogenesis of Larix leptolepis. Plant Cell Tiss Org 100(1):21–29
Zhao YN, Luo LL, Xu JS, Xin PY, Guo HY, Wu J, Bai L, Wang GD, Chu JF, Zuo JR, Yu H, Huang X, Li JY (2018) Malate transported from chloroplast to mitochondrion triggers production of ROS and PCD in Arabidopsis thaliana. Cell Res 28(4):448–461
Zhou T, Yang XY, Guo K, Deng JW, Xu J, Gao WH, Lindsey K, Zhang XL (2016) ROS homeostasis regulates somatic embryogenesis via the regulation of auxin signaling in Cotton. Mol Cell Proteomics 15(6):2108–2124
Acknowledgements
The authors thank Dr. Zhengquan Wang of the Northeast Forestry University, Harbin, China, for comments that improved an earlier draft of this paper.
Author information
Authors and Affiliations
Contributions
Y. L and S. HL conceived and designed the study. Y. L and Z. DY collected plant materials and prepared SE samples for analysis. W. H and Z. DY analyzed the results for experiments. Y. L, Z. DY and W. H contributed to the writing of the manuscript and data analyses. F. JL and S. HL revised the manuscript. All authors read and approved the final manuscript.
Corresponding authors
Additional information
Corresponding editor: Yanbo Hu.
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
The original online version of this article was revised: In this article Ling Yang should have been denoted as a corresponding author.
Project funding: This work was supported by the Fundamental Research Funds for the Central Universities (2572018BW02), the Innovation Project of State Key Laboratory of Tree Genetics and Breeding (2016C01), the National Key R & D Program of China (2017YFD0600600) and the National Natural Science Foundation of China (31400535 and 31570596).
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Wang, H., Zhang, D., Fernández-Lorenzo, J.L. et al. Reactive oxygen species, nitric oxide and plant cell death associated with caspase-like protease activity during somatic embryogenesis in Fraxinus mandshurica. J. For. Res. 33, 1005–1017 (2022). https://doi.org/10.1007/s11676-021-01392-y
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11676-021-01392-y