Abstract
The phase equilibria in the La2O3-Yb2O3 system were investigated using differential thermal analysis, and the phase transformations temperatures were verified. Microstructure investigation using scanning electron microscopy confirmed the presence of eutectoid reactions in the system. The heat capacity of LaYbO3 with a perovskite structure was measured in the temperature range from 250 to 1250 K using differential scanning calorimetry. The experimental results of the present work as well as reliable literature data were used to derive a thermodynamic description of the La2O3-Yb2O3 system. Isothermal sections of the ZrO2-La2O3-Yb2O3 system were constructed based on x-ray and microstructural investigations. The tie-lines change from Pyrochlore + \({\text{Yb}}_{2} {\text{O}}_{3} \_{\text{C}}\) to \({\text{LaYbO}}_{3} \_{\text{Perovskite}}\) + Fluorite was explained by a solid-state reaction occurring in the range between 1673 and 1873 K. The heat effect observed at 1858 K using DTA confirms the phase transformation, and its temperature was established more precisely at 1808 ± 15 K using stepwise heat treatment.
Similar content being viewed by others
1 Introduction
Y2O3 stabilized ZrO2 (YSZ) is widely used as a thermal barrier coating (TBC) applied in the aerospace and power plant industry. A metastable tetragonal phase (t′) containing 6-8 mass% Y2O3, exceeding maximal solubility in the tetragonal phase according to the phase diagram, is the material presently used for TBC. However, the operating temperature of TBC based on 7YSZ is limited to temperatures below 1473 K due to the separation of the metastable t′ phase into equilibrium phase assemblage tetragonal + cubic (fluorite structure) with further transformation of the equilibrium t phase to the monoclinic phase.[1] Therefore, it is necessary to increase the thermal insulation capability and stability of candidate-materials for TBC. A further decrease in the thermal conductivity of the TBC material and its sintering resistance compared to YSZ helps to increase the thermal insulation ability of TBC. Experimental investigations show that the addition of rare earths is one of the most promising methods to meet the needs of the next generation engine.[2,3,4,5]
Candidate materials for the new TBC can be divided into two main groups: the first group is based on co-doping of YSZ with one or more rare-earths (RE2O3), the second group includes the zirconates with a pyrochlore structure.[2,3,6] Among the rare-earth oxides, it was reported[4] that co-doping of YSZ with La2O3 increased its resistance to sintering, reduced thermal conductivity, and increased structural stability. It is worthy to note that the formation of the La2Zr2O7 secondary phase with a pyrochlore structure results in a significant suppression of densification. La2Zr2O7 pyrochlore was proposed as a promising TBC material;[2,6,7] therefore, its properties and stability in thermal cycling were investigated. Moreover, according to experimental data, the phase stability of singly doped zirconia at a constant dopant concentration (7.6%) shows a strong dependence on the cation size, being optimal for Yb and Y, and declining both for larger and smaller cations.[2] It was found that co-doping with two different rare-earths having larger and smaller ionic radius decreases thermal conductivity of the material. Co-doping of pyrochlore with different rare-earth elements was then suggested to further decrease its thermal conductivity and to modify the thermal expansion coefficient. The rare-earth oxide doped pyrochlores La2-xYbxZr2O7 have a lower thermal conductivity than un-doped La2Zr2O7 (pyrochlore) and Yb2Zr2O7 (fluorite).[8] A substantial improvement in the mechanical properties of materials with intermediate compositions has also been shown.[8] Thus, Yb2O3 is one of the best stabilizers when La2O3 is used as a dopant in the ZrO2-based materials.
Phase diagrams of the ZrO2-La2O3-Yb2O3 system were not investigated so far except for limited information for the La2Zr2O7-Yb2Zr2O7 section.[8] The knowledge of the phase diagram of the ZrO2-La2O3-Yb2O3 system and the thermal properties of the phases present in the system will expand the possible composition range of materials with low thermal conductivity for TBC application and, thus, to increase the efficiency of gas turbines.
2 Literature Survey
2.1 La2O3-Yb2O3 Binary System
The phase relations in the La2O3-Yb2O3 system were studied only by Rouanet et al.[9] and Coutures et al.[10] so far. According to the literature data,[9,10] the A and H hexagonal phases dissolve up to 20 mol.% of Yb2O3, while the cubic phase X dissolves up to 90 mol.% of Yb2O3. The \({\text{Yb}}_{2} {\text{O}}_{3} \_{\text{C}}\) cubic phase has a rather limited solubility of La2O3 which reaches a maximum (10 mol.% La2O3) in the peritectic reaction \({\text{L}} + {\text{Yb}}_{2} {\text{O}}_{3} \_{\text{C}} \to {\text{X}}\) at 2553 K. Invariant reactions involving the high-temperature hexagonal phase H were not shown in the phase diagram, although it was found both in unary Yb2O3 and La2O3 systems.[11] The phase diagram of the La2O3-Yb2O3 system according to Coutures et al.[10] is shown in Fig. 1(a). According to both experimental studies,[9,10] an intermediate compound LaYbO3 with a perovskite structure is stable up to 2313 K and then transforms congruently to the high-temperature phase X. Its crystal structure information as well as for all solid phases of the quasi-binary systems are listed in Table 1. According to Rouanet et al.,[9] the perovskite phase has homogeneity ranges from 45 to 65 mol.% of Yb2O3, while Coutures et al.[10] reported an extension of the LaYbO3 homogeneity range only towards Yb2O3 composition. Two invariant reactions were detected:[9,10] \({\text{X}} \to {\text{Per}} + {\text{A}}\) at 2093 K and \({\text{X}} \to {\text{Per}} + {\text{Yb}}_{2} {\text{O}}_{3} \_{\text{C}}\) at 2203 K. The phase X melts congruently at a minimal temperature of 2403 K. Experimental thermodynamic information is available only for the perovskite compound. The enthalpy of formation of perovskite LaYbO3 formed from oxides was reported based on drop-solution calorimetry measurements.[12] It should be noted that several phase boundaries were tentatively shown in the phase diagram of the La2O3-Yb2O3 system.[9,10] Therefore, to derive thermodynamic parameters of the La2O3-Yb2O3 system, additional study of phase equilibria and determination of thermodynamic values are necessary.
2.2 ZrO2-Yb2O3 Binary System
The experimental investigations of phase equilibria in the ZrO2-Yb2O3 system were performed by several research groups.[13,14,15] The phase diagram of the ZrO2-Yb2O3 system is characterized by a wide homogeneity range of the fluorite phase and the presence of the stoichiometric compound Zr3Yb4O12 (δ-phase). The δ-Zr3Yb4O12 phase is characterised by a trigonal crystal structure, which is a fluorite superstructure that transforms into a fluorite structure at a temperature of 1903 K. Wang[15] took into account the data of Karaulov and Zoz[16] that the δ-phase has a homogeneity range from 37 to 45 mol.% of Yb2O3. However, these results were not confirmed by Lakiza et al.[17] It should be mentioned that there are substantial discrepancies between the results of Wang[15] and the results of Lakiza et al.[17] in the composition of the \({\text{Yb}}_{2} {\text{O}}_{3} \_{\text{C}}\) phase, which is in equilibrium with δ-Zr3Yb4O12 and in equilibrium with the fluorite phase. Additionally, there are very large differences in the compositions of the fluorite phase in equilibrium with \({\text{Yb}}_{2} {\text{O}}_{3} \_{\text{C}}\) determined by Wang[15] and determined in works of Stubican et al.[14] and Lakiza et al.[17] which indicated a lower content of Yb2O3 in fluorite then determined by Wang.[15] Rouanet[13] indicated even higher concentration of Yb2O3 in fluorite phase in equilibrium with \({\text{Yb}}_{2} {\text{O}}_{3} \_{\text{C}}\) than Wang.[15] Besides sub-solidus relations in the Yb2O3-rich region, Rouanet[13] studied the liquidus curve of the ZrO2-Yb2O3 system using thermal analysis and found a maximum and eutectic reaction \({\text{L}} \to {\text{Fluorite}} + {\text{Yb}}_{2} {\text{O}}_{3} \_{\text{H}}\). In spite the ZrO2-Yb2O3 system was studied in several works, the results obtained are contradictory, and the phase diagram is still not very well established.
Experimental thermodynamic information is limited to measurements of the enthalpy increment for fluorite and δ-phase,[18] the enthalpy of formation of the δ-phase and fluorite solid solutions in the range from 25 to 57 mol.% of Yb2O3 obtained by drop-solution calorimetry and vapour pressure data at 2400 K by Stolyarova et al.[19]
The first thermodynamic assessment based only on phase equilibrium data of Rouanet[13] and Stubican et al.[14] was performed by Jacobson et al.[20] A more advanced thermodynamic modelling of this binary system was performed by Wang[15] based on his own results (phase equilibria study and calorimetric measurements of the enthalpy increment for the fluorite and Zr3Yb4O12 phases) as well as data from literature.[13,14,16,21] A recent re-assessment of the ZrO2-Yb2O3 system was published by Fabrichnaya et al.[22] where the thermodynamic description[23] was modified to be compatible with other binary systems in the description of the ZrO2-Yb2O3-Al2O3 system to reproduce experimental data on the liquidus surface. Available experimental data[18,19] were also taken into account. The calculated phase diagram is shown in Fig. 1(b).
2.3 ZrO2-La2O3 Binary System
The phase diagram of the ZrO2-La2O3 system was constructed in the work of Rouanet.[13] Additional experimental study of the system was performed by Wang.[15] Thermodynamic data (enthalpy of formation, standard entropy and heat capacity) are available for an intermediate compound La2Zr2O7 with a pyrochlore structure[15,24,25,26] as well as the melting enthalpy of this compound.[26] A detailed review of the experimental data can be found elsewhere.[27,28] Thermodynamic descriptions for the system were published in several works.[27,28,29] The phase diagram of the ZrO2-La2O3 system calculated by Fabrichnaya et al.[29] is accepted in the present study and shown in Fig. 1(c).
3 Experimental Procedure
Zr(CH3COO)4 (99.99%, Sigma-Aldrich), La(NO3)3·6H2O (99.99%, Alfa Aesar) and Yb(NO3)3·6H2O (99.9%, Alfa Aesar) were used as the starting chemicals. The list of the sample compositions investigated in the present work is presented in Table 2. At the first stage, the chemicals were separately dissolved in distilled water and then mixed according to the given ratios. The thus obtained precursor solution was added dropwise at a low speed (around 1 ml min−1) into a large beaker containing about 500 ml of distilled water. The pH value was maintained above 9.0 by adding ammonium hydrate (ammonia aqueous solution). During dropping and stirring, the precipitation was visible by getting a white hazy suspension. The obtained suspension was heated up and kept at 333 K for 1-2 h leading to a better coagulation of the particles and the evaporation of most of the water. After filtration and drying at 353 K, the powder was calcined at 1073 K for 3 h in air. It should be noted that filtrate and precipitate after filtration were analyzed by Inductively Coupled Plasma spectrometry (ICP-OES) with an experimental accuracy of ± 2 at.%. The resulting powder was ball-milled, pressed into pellets, and then heat treated in air atmosphere in Pt-crucibles using NABERTHERM furnace to reach an equilibrium state. The sintered samples were consequently analyzed by XRD, scanning electron microscope (SEM) combined with energy dispersive x-ray spectroscopy (EDX).
XRD patterns of the powdered specimen were recorded using the Präzisionsmechanic diffractometer (CuKα radiation; Freiberg, Germany). The lattice parameters for the phases and their volume fractions were calculated by Rietveld analysis using MAUD software.[30]
The microstructures of the sintered samples were examined by SEM (Leo1530 GEMINI) using back scattered electrons imaging technique (BSE), and energy dispersive x-ray spectroscopy (EDX Bruker AXS Mikroanalysis GmbH) was employed to determine the compositions of the phases (± 3 mol.% REO1.5) in the equilibrium state.
Two different devices by SETARAM (France) were used for differential thermal analysis (DTA): SETSYS EVOLUTION 1750 and 2400 (TG-DTA). The melting of sample materials was investigated using the SETSYS EVOLUTION 2400 (TG-DTA) in W crucibles in He atmosphere at temperatures up to 2173 K. The heating rate in both instruments was 20 K min−1 up to 1473 K and then 10 K min−1; the cooling rate was of 30 K min−1, which was also the same for both instruments. Temperature calibration of the SETSYS EVOLUTION 1750 was performed using the melting points of Al, Ag, Au, Cu and Ni, whereas the temperature calibration of the SETSYS EVOLUTION 2400 was made using the melting points of Al, Au, and Al2O3, and the temperature of transformation of LaYO3 to the monoclinic phase B.[31]
The heat capacity of the samples heat treated at 1523 K and consisting of single-phase LaYbO3 (perovskite structure) was determined using differential scanning calorimetry (DSC). The measurements in the temperature range from 235 to 675 K were carried out using the device DSC 8000 (Perkin Elmer, Pt/Rh crucible, Ar flow, heating rate 10 K/min), whereas the measurements in the temperature range from 235 to 675 K were divided into small intervals 100-150 K. The heat capacity measurements of the samples in the temperature range from 623 to 1220 K were performed covering the whole temperature range using the device DSC Pegasus 404C (NETZSCH, Pt/Rh crucible, Ar flow, heating rate 10 K/min). The classical three-step method (continuous method) with a constant heating rate was used to measure the heat capacities, and the calorimeters were calibrated using a certified sapphire standard material. The masses and radii of the sample pellets were kept the same as for the standard material c.a. 85 mg and 5 mm. The measurements of two different samples were repeated twice with a maximal uncertainty of 3%. It should be mentioned that the CP measurements at high temperatures using DSC equipment are intrinsically less reliable due to the increase in heat radiation which decreases the measured signal. This effect was considered during the analytic evaluation of the experimental data, which were fitted using the Maier–Kelley equation.[32] In order to exclude any phase transformations during the heat capacity measurements, XRD analysis of the obtained samples were performed before and after the heat capacity measurements.
4 Results and Discussion
Sample compositions were selected for experimental investigations to verify the phase diagram of the La2O3-Yb2O3 system (Fig. 1) and to study the ZrO2-La2O3-Yb2O3 system which was not investigated so far. The results of XRD investigation with subsequent Rietveld refinement for the samples after heat treatments are listed in Table 2. The nominal compositions of the samples and the results of experimental measurements using ICP-OES and SEM/EDX methods are also presented in Table 2.
4.1 Experimental Data for the La2O3-Yb2O3 System
The experimental results obtained for the sample LYb-B1 containing 30 mol.% of Yb2O3 are presented in Fig. 2. Two phases \({\text{La}}_{2} {\text{O}}_{3} \_{\text{A}}\) and \({\text{LaYbO}}_{3} \_{\text{Per}}\) were indicated by XRD after heat treatment at 1873 K (see Fig. 2a). The results obtained are in agreement with the published phase diagram. The SEM image of the sample after heat treatment at 1873 K, shown in Fig. 2(c), indicate a two-phase area of \({\text{LaYbO}}_{3} \_{\text{Per}}\) (light gray contrast) and \({\text{La}}_{2} {\text{O}}_{3} \_{\text{A}}\) (dark gray contrast). According to EDX measurements, the composition of these two phases were correspondingly 7 mol.% of Yb2O3 in \({\text{La}}_{2} {\text{O}}_{3} \_{\text{A}}\) and 48 mol.% of Yb2O3 in Perovskite. The sample LYB-B1 was investigated using DTA up to 2333 K, and three thermal effects were observed on heating (see Fig. 2b). The first effect at 2102 K can be attributed to the reaction A + Per \(\to\) H, the second one at 2123 K—reaction H + Per \(\to\) X, and the third one at 2147 K is X-phase solvus. It should be noted that only one effect was observed on cooling due to the fact that all three reverse transformations occurred in the narrow temperature range. The observed undercooling effect was not very large. The microstructure investigation of the sample after DTA indicated a eutectoid-type structure (see Fig. 2d). The measured phase compositions after DTA showed that the La2O3-rich phase contained up to 16 mol.% of Yb2O3, a homogeneity range of Perovskite extended remarkably to La2O3 direction (35 mol.% of Yb2O3), and the eutectoid composition was 25 mol.% of Yb2O3.
XRD, DTA and SEM/EDX data for the sample LYb-B1. (a) XRD after annealing at 1523, 1673, and 1873 K; (b) DTA heating and cooling curves; (c) microstructure of the sample before DTA; and (d) microstructure of the sample after DTA. In both microstructures, dark phase is \({\text{La}}_{2} {\text{O}}_{3} \_{\text{A}}\) and white phase is \({\text{LaYbO}}_{3} \_{\text{Per}}\)
For the sample LYb-B2 containing 50 mol.% of Yb2O3, the results obtained are presented in Fig. 3. The single-phase Perovskite was identified by XRD after heat treatment at 1673 and 1873 K (see Fig. 3a). Two peaks were observed on DTA heating curve for this sample at 2290 and 2322 K (see Fig. 3b). This could be explained by the extension of the homogeneity range in La2O3-rich compositions at high temperature, as indicated by the analysis of the LYb-B2 sample after DTA. According to ICP-OES, this sample was slightly enriched in La2O3 (51 mol.%) compared to the composition of LaYbO3, and these two peaks were caused by the phase transformations Per \(\to\) Per + X and Per + X \(\to\) X. The microstructure investigation of this sample after DTA showed the presence of small La2O3 grains in the LaYbO3 matrix, while before DTA after heat treatment at 1873 K, a single-phase \({\text{LaYbO}}_{3} \_{\text{Per}}\) was present, as indicated by XRD and SEM/EDX (see Fig. 3a and c). That means that the temperature of the congruent transformation for Perovskite to the X-phase was slightly higher than 2322 K.
XRD, DTA and SEM/EDX data for the sample LYb-B2. (a) XRD after heat treatments at 1673 and 1873 K; (b) DTA heating and cooling curves; (c) microstructure of the sample before DTA; (d) microstructure of the sample after DTA. After DTA, small \({\text{La}}_{2} {\text{O}}_{3} \_{\text{A}}\) dark grains were observed in the \({\text{LaYbO}}_{3} \_{\text{Per}}\) matrix
The results obtained for the sample LYb-B3 containing 70 mol.% of Yb2O3 are presented in Fig. 4. Two phases \({\text{LaYbO}}_{3} \_{\text{Per}}\) and \({\text{Yb}}_{2} {\text{O}}_{3} \_{\text{C}}\) were detected using XRD after heat treatment at 1673 and 1873 K (Fig. 4a). The compositions of these two phases measured by EDX after heat treatment at 1873 K were 54 and 96 mol.% of Yb2O3, correspondently in the Perovskite and Yb2O3-C phases. According to the phase diagram of the La2O3-Yb2O3 system, there is a high-temperature hexagonal phase, which reversibly transforms to the C phase on cooling. Therefore, both the H and X phases should be stable in the Yb2O3-enriched compositions at high temperature. In the present work, the temperature of the eutectoid reaction X(or H) \(\to\) Per + C was determined using DTA to be 2295 K (Fig. 4b), which is substantially higher compared to the literature data of 2198 K.[9] It should be noted that there was large undercooling and the reverse transformation was observed at 2170 K. Due to the fact that the transformation temperature was determined on cooling,[9,10] this could be the reason of the inconsistency between the results of the present work and the literature ones.[9,10] The microstructure of the sample after DTA confirms the eutectoid structure (Fig. 4d). The eutectoid composition was measured to be 60 mol.% of Yb2O3. The compositions of the Perovskite and \({\text{Yb}}_{2} {\text{O}}_{3} \_{\text{C}}\) phases after DTA were 55 mol.% of Yb2O3 in Perovskite phase and 76 mol.% of Yb2O3 in \({\text{Yb}}_{2} {\text{O}}_{3} \_{\text{C}}\) phase.
XRD, DTA and SEM/EDX data for the sample LYb-B3. (a) XRD after annealing at 1673 and 1873 K; (b) DTA heating and cooling curves; (c) microstructure of the sample before DTA; and (d) microstructure of the sample after DTA. In both microstructures, white phase is \({\text{Yb}}_{2} {\text{O}}_{3} \_{\text{C}}\) and dark phase is \({\text{LaYbO}}_{3} \_{\text{Per}}\)
The sample LYb-B2 heat treated at 1873 K was used for the heat capacity measurements using DSC in the range of 250-1250 K. The obtained data were fitted using Maier–Kelley equation as follows
It should be noted that at temperatures above 1100 K, the measured heat capacity demonstrates an unrealistic decrease with temperature. Therefore, only data below 1100 K were used to derive Eq 1. The experimental data on the heat capacity, calculated lines using the Eq 1 and using the Neumann–Kopp rule are presented in Fig. 5. It should be mentioned that the heat capacity calculated using the Neumann–Kopp rule was slightly lower at high temperature than the calculated using Eq 1, but both were within experimental uncertainty.
Heat capacity measurements of \({\text{LaYbO}}_{3} \_{\text{Per}}\) along with calculation based on Neumann–Kopp rule and fitted curve. Dulong–Petit line is correspond to the value n3R, where R is the gas constant and n is the number of atoms in the formula unit, namely five for \({\text{LaYbO}}_{3} \_{\text{Per}}\)
New data about phase equilibria and heat capacity measurements for the \({\text{LaYbO}}_{3} \_{\text{Per}}\) compound were used in the assessment of thermodynamic parameters of the La2O3-Yb2O3 system.
4.2 Thermodynamic Modelling of the La2O3-Yb2O3 System
To describe solid solutions in the La2O3-Yb2O3 system, the compound energy formalism described by Hillert[33] was used. The homogeneity ranges of the intermediate compound LaYbO3-Per were considered, and this phase was also described using the compound energy formalism.
The Gibbs energy of the formation of the solution phases, modelled by two sublattices, is expressed as
where Gij is the Gibbs energy of end-member compound ij, \(Y_{i}^{s}\) is the mole fraction of constituent i on the sublattice s, αs is the stoichiometric number of sites on the sublattice s per mole of formula unit of a phase, and \(\Delta G^{ex}\) is the excess Gibbs energy of mixing expressed as
Binary interaction parameters \(L_{i,k}^{s}\) between i and k species on the sublattice s were expressed by Redlich–Kister polynomials
Interaction parameters \(L_{j,m}^{t}\) between j and m species on the sublattice t were described in the same way. The term \(\Delta G_{{}}^{ex,h - 0}\) is the high-order contribution to the excess Gibbs energy. The first term in Eq 2 is the surface reference, while the second and third terms are the ideal entropy of mixing for each sublattice multiplied by − T.
The optimization of the thermodynamic parameters was performed using the phase equilibria data obtained in the present work and from the literature.[9,10] The heat capacity measured in the present study for \({\text{LaYbO}}_{3} \_{\text{Per}}\) was introduced in the description of the LaYbO3 end-member. The drop solution calorimetry data[12] were also taken into account during optimization. The thermodynamic parameters obtained for the system La2O3-Yb2O3 are listed in Table 3.
The calculated phase diagram of the La2O3-Yb2O3 system is presented in Fig. 6. The calculated information on invariant reactions is compared with experimental data in Table 4. The calculations showed two eutectoid reactions in the La2O3-enriched compositions and two in the Yb2O3-enriched compositions. In,[9,10] the A + H two-phase field was shown as tentative in the La2O3-rich compositions, while the H phase was not considered in the Yb2O3-rich compositions. In the present work, the high-temperature hexagonal phase H was considered both in the La2O3 and Yb2O3 bounding systems using the thermodynamic functions from work of Zinkevich.[11] The reason why two reactions H ↔ Per + C and X ↔ Per + H were obtained in the calculation, unlike the one X ↔ Per + C reaction in,[9] is because the H-phase in the Yb2O3-rich compositions was considered in the calculations. The calculated temperatures of H ↔ Per + C and X ↔ Per + H are close to each other, and this could explain the fact that DTA investigation indicated one thermal effect on heating, while two thermal effects were observed on cooling due to the separation of the effects in course of large undercooling.
It should be noted that the enthalpy of formation of \({\text{LaYbO}}_{3} \_{\text{Per}}\) from oxides at 298.15 K derived by CALPHAD approach appeared to be more negative (− 21.31 kJ/mol) than that obtained by drop-solution calorimetry (− 12.3 kJ/mol). The enthalpy of formation for LaYO3 perovskite was measured using drop-solution calorimetry equal to − 50.87 kJ/mol,[34] which was probably overestimated, since the CALPHAD modelling resulted in a value of − 42.44 kJ/mol. The compound LaYO3, having the same crystal structure as LaYbO3, while it is less stable transforming into the monoclinic structure B at 1863 K. Therefore, it is possible that the value for LaYbO3 is underestimated. Values for both compounds should be verified in future experiments.
4.3 Experimental Data for the ZrO2-Yb2O3-La2O3 Ternary System
The phase relations in the ZrO2-Yb2O3-La2O3 ternary system were investigated by sample equilibration and further characterization. The compositions were selected at three different ZrO2 content and three different ratios La/(Yb + La). Additional sample compositions were selected to clarify phase relations which were absent in the first series of samples. The samples of the system were annealed at temperatures of 1523, 1673 and 1873 K. The phases present in the samples were identified using XRD, and the phase compositions were measured using SEM/EDX (Table 2). It should be noted that extension into the ternary system was found in the following phases: F-ZrO2, La2Zr2O7 pyrochlore, \({\text{Yb}}_{2} {\text{O}}_{3} \_{\text{C}}\), and LaYbO3 perovskite. It should be noted that ternary extension in these phases was also observed in a similar system ZrO2-La2O3-Y2O3.[35,36]
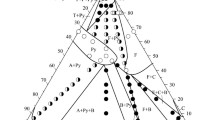
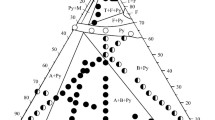
Based on experimental phase equilibrium information, isothermal sections at 1523, 1673 and 1873 K were constructed, Fig. 7. At 1523 K, three three-phase regions, Pyr + Per + \({\text{La}}_{2} {\text{O}}_{3} \_{\text{A}}\), Pyr + Per + \({\text{Yb}}_{2} {\text{O}}_{3} \_{\text{C}}\), and Pyr + Flu + \({\text{Yb}}_{2} {\text{O}}_{3} \_{\text{C}}\), were determined based on XRD results confirmed by SEM/EDX investigations. Although in Fig. 7(a), the solid solution ranges were predicted in a rather qualitative way, since a very fine microstructures were observed for the samples heat treated at 1523 K. Therefore, ternary extensions of the pyrochlore and perovskite phases are not shown. Also, two two-phase regions, Pyr + Flu and Pyr + \({\text{Yb}}_{2} {\text{O}}_{3} \_{\text{C}}\), were detected. Another two three-phase regions (Pyr + Flu + Tet and Flu + C + δ) were predicted based on the phase diagram analysis of the binary and ternary systems. In comparison to the isothermal section at 1523 K, the phase relations at 1673 K are similar. However, with further increasing of temperature, the phase relations changed. At 1873 K, different three-phase regions Pyr + Per + Flu and Per + Flu + C were detected. Comparing two isothermal sections at 1673 and 1873 K, it can be observed that the tie-line between Pyrochlore and \({\text{Yb}}_{2} {\text{O}}_{3} \_{\text{C}}\) at 1673 K changed to the tie-line between Fluorite and \({\text{LaYbO}}_{3} \_{\text{Per}}\) at 1873 K. Therefore, in this temperature range, a solid-state phase transformation Per + Flu \(\to\) Pyr + C should occur. It should be noted that the same transformation was observed between 1673 and 1873 K in the similar system ZrO2-La2O3-Y2O3.[36]
To find the temperature of this transformation, the samples ZLYb-8,-9,-10 were investigated using DTA. The thermal effect at a temperature of about 1858 ± 10 K was observed in all three samples, as shown in Fig. 8. The microstructures of these samples obtained after hear treatment at 1673 and 1873 K are presented in Fig. 9. It should be noted that the temperature obtained by DTA could be slightly higher than the real one due to the overheating effect. Therefore, in order to clarify the temperature of this transformation, the samples were stepwise heat treated and followed by XRD examination. It was then indicated that the transformation occurred between 1793 and 1823 K. Therefore, the temperature of the solid-state transformation Per + Flu \(\to\) Pyr + C was established at 1808 ± 15 K.
5 Conclusions
The phase equilibria in the La2O3-Yb2O3 system were investigated and the phase transformations temperatures were verified. An extension of the homogeneity range of the LaYbO3 perovskite phase into both La2O3- and Yb2O3-rich compositions was found. The measured temperature of the X/H ↔ A + Per eutectoid reaction and congruent transformation of \({\text{LaYbO}}_{3} \_{\text{Per}}\) to the X phase are in reasonable agreement with the previous results.[9,10] The temperature of the eutectoid reaction X/H \(\leftrightarrow\) Per + C in the Yb2O3-rich compositions was determined using DTA at substantially higher temperature than that in Ref 9 and 10. Probably the difference can be explained by the fact that in contrast to the present study the transformation temperature was determined on cooling mode in Ref 9 and 10. The heat capacity of \({\text{LaYbO}}_{3} \_{\text{Per}}\) was measured in the range between 250 and 1250 K.
The experimental data obtained in the present work along with data from the literature were used to optimize thermodynamic parameters of the La2O3-Yb2O3 system. The calculated phase diagram is in reasonable agreement with the experimental data.
Isothermal sections of the ternary system ZrO2-La2O3-Yb2O3 were constructed at 1523, 1673 and 1873 K based on experimental data obtained in the present study for the first time. The solid-state reaction Pyr + C \(\to\) Per + Flu was found at the temperature of 1808 ± 15 K.
The obtained information about phase diagram of the ZrO2-La2O3-Yb2O3 system can be further used to select compositions for possible TBC materials. Also, the obtained thermodynamic description of the La2O3-Yb2O3 system can be combined with the available description of the ZrO2-La2O3 and ZrO2-Yb2O3 systems to develop a preliminary description of the ternary system. The ternary parameters describing the extension of the homogeneity ranges of the phases into the ternary system should be optimized based on the experimental information about phase relations obtained in the present study. The calculations based on data at 1673 and 1873 K could provide better prediction of the phase compositions at 1523 K.
References
N.R. Rebollo, O. Fabrichnaya, and C.G. Levi, Phase Stability of Y+Gd Co-doped Zirconia, Z. Metall., 2003, 94, p 163-170
C.G. Levi, Emerging Materials and Processes for Thermal Barrier Systems, Curr. Opin. Solid State Mater. Sci., 2004, 8, p 77-91
R. Vaßen, M.O. Jarligo, T. Steinke, D.E. Mack, and D. Stöver, Overview on Advanced Thermal Barrier Coatings, Surf. Coat. Technol., 2010, 205, p 938-942
Y. Liu, Y.F. Gao, S.Y. Tao, X.M. Zhou, and H.J. Luo, La2O3-Modified YSZ Coating: High-Temperature Stability and Improved Thermal Barrier Properties, Surf. Coat. Technol., 2009, 208, p 1014-1019
J.M. Cairnay, N.R. Rebollo, M. Rühle, and C.G. Levi, Phase Stability of Thermal Barrier Coatings: A Comparative Study of Y and Yb Additions, Z. Metall., 2007, 98, p 1177-1187
J. Wu, X. Wie, N.P. Padture, P.G. Klemens, M. Gell, E. Garcia, P. Miranzo, and M.I. Osendi, Low-Thermal Conductivity Rare-Earth Zirconates for Potential Thermal-Barrier-Coating Applications, J. Am. Ceram. Soc., 2002, 85, p 3031-3035
S.T. Aruna, C. Sanjeeviraja, N. Balaji, and N.T. Manikandanath, Properties of Plasma Sprayed La2Zr2O7 Coating Fabricated from Powder Synthesized by a Single-Step Solution Combustion Method, Surf. Coat. Technol., 2013, 219, p 131-138
X. Ren, C. Wan, M. Zhao, J. Yang, and W. Pan, Mechanical and Thermal Properties of Fine-Grained Quasi-Eutectoid (La1-xYbx)2Zr2O7 Ceramics, J. Eur. Ceram. Soc., 2015, 35, p 3145-3154
A. Rouanet, J. Coutures, and M. Foex, Study of High Temperature Equilibrium Phase Diagram of the La2O3-Yb2O3 System, J. Solid State Chem., 1972, 4, p 219-222
J. Coutures, A. Rouanet, R. Verges, and M. Foex, High-Temperature Study of Systems Forms by Sesquioxides of Lanthane and Sesquioxides of Lanthanides, J. Solid State Chem., 1976, 17, p 171-182
M. Zinkevich, Thermodynamics of Rare Earth Sesquioxides, Prog. Mater Sci., 2007, 52, p 597-647
J. Qi, X. Guo, A. Mielewczyk-Gryn, and A. Navrotsky, Formation Enthalpies of LaLn’O3 (Ln’=Ho, Er, Tm and Yb) Interlantanide Perovskites, J. Solid State Chem., 2015, 227, p 150-154
A. Rouanet, Contribution to Study of Zirconium-Oxides Systems of Lanthanides Close to Melting Point, Rev. Int. Ht. Temper. Refract., 1971, 8, p 161-180
V.S. Stubican, G.S. Corman, J.R. Hellmann, and G. Senft, Phase relationships in some ZrO2 systems, Advances in Ceramics, Vol. 12 Science and Technology of Zirconia II, N. Claussen, M. Ruehle, and A.H. Heuer, Ed., American Ceramic Society, Columbus, 1984, p 96-106
C. Wang, Experimental and Computational Phase Studies of the ZrO2-Based Systems for Thermal Barrier Coatings. Ph.D. Thesis (University of Stuttgart, Germany, 2006)
A.G. Karaulov and E.I. Zoz, Phase Formation in the ZrO2-HfO2-Gd2O3 and ZrO2-HfO2-Yb2O3 Systems, Refract. Ind. Ceram, 1999, 40, p 479-483
S.M. Lakiza, V.P. Red’ko, and L.M. Lopato, Isothermal Sections at 1250 and 1650°C, Powder Metal. Metal. Ceram., 2008, 47, p 202-210
P. Simoncic and A. Navrotsky, Systematics of Phase Transition and Mixing Energetics in Rare Earth, Yttrium, and Scandium Stabilized Zirconia and Hafnia, J. Am. Ceram. Soc., 2007, 70, p 2143-2150
V.L. Stolyarova, S.I. Lopatin, O.B. Fabrichnaya, and S.M. Shugurov, Mass Spectrometric Study of Thermodynamic Properties in the Yb2O3-ZrO2 System at High Temperatures, Rapid Commun. Mass Spectrom., 2014, 28, p 109-114
N.S. Jacobson, Z.-K. Liu, L. Kaufman, and F. Zhang, Calculation of Phase Equilibria in the Y2O3-Yb2O3-ZrO2 System, Electrochem. Soc. Proc., 2002, 5, p 1-8
M. Gonzalez, C. Moure, J.R. Jurado, and P. Duran, Solid-State Reaction, Microstructure and Phase Relations in the ZrO2-Rich Region of the ZrO2-Yb2O3 System, J. Mater. Sci., 1993, 28, p 3451-3456
O. Fabrichnaya, S.M. Lakiza, M.J. Kriegel, J. Seidel, G. Savinykh, and G. Schreiber, New Experimental Investigations of Phase Relations in the Yb2O3-Al2O3 and ZrO2-Yb2O3-Al2O3 Systems and Assessment of Thermodynamic Parameters, J. Eur. Ceram. Soc., 2015, 35, p 2855-2871
O. Fabrichnaya and H.J. Seifert, Thermodynamic Assessment of the ZrO2-Yb2O3-Al2O3 System, CALPHAD, 2010, 34, p 206-214
D. Sedmidubsky, O. Benes, and R.J.M. Konings, High Temperature Heat Capacity of Nd2Zr2O7 and La2Zr2O7 Pyrochlores, J. Chem. Thermodyn., 2005, 37, p 1098-1103
M. Bolech, E.H.P. Cordfunke, A.C.G. Van Genderen, R.R. Van Der Laan, F.J.J.G. Janssen, and J.C. Van Miltenburg, The Heat Capacity and Derived Thermodynamic Function of La2Zr2O7 and Ce2Zr2O7 from 4 to 1000 K, J. Phys. Chem. Solids, 1997, 58, p 433-439
A.V. Radha, S.V. Ushakov, and A. Navrotsky, Thermochemistry of Lanthanum Zirconate Pyrochlore, J. Mater. Res., 2009, 24, p 3350-3357
O. Fabrichnaya, S. Lakiza, C. Wang, M. Zinkevich, and F. Aldinger, Assessment of Thermodynamic Functions in the ZrO2-La2O3-Al2O3 System, J. Alloys Compd., 2008, 453, p 271-281
C. Wang, O. Fabrichnaya, M. Zinkevich, Y. Du, and F. Aldinger, Experimental Study and Thermodynamic Modelling of the ZrO2-LaO1.5 System, CALPHAD, 2008, 32(1), p 111-120
O. Fabrichnaya, G. Savinykh, and G. Schreiber, Phase Relations in the ZrO2-La2O3-Y2O3-Al2O3 System: Experimental Study and Thermodynamic Modelling, J. Eur. Ceram. Soc., 2013, 33(1), p 37-49
L. Lutterotti, S. Matthies, and H.R. Wenk, MAUD: A Friendly Java Program for Material Analysis Using Diffraction, CPD Newslett., 1999, 21, p 14-15
O. Fabrichnaya, M.J. Kriegel, D. Pavlyuchkov, J. Seidel, A. Dzuban, G. Savinykh, and G. Schreiber, Heat Capacity for the Eu2Zr2O7 and Phase Relations in the ZrO2-Eu2O3 System: Experimental Studies and Calculations, Thermochim. Acta, 2013, 558, p 74-82
C.G. Maier and K.K. Kelley, An Equation for the Representation of High-Temperature Heat Content Data, J. Am. Chem. Soc., 1932, 54, p 3242-3246
M. Hillert, The Compound Energy Formalism, J. Alloys Compd., 2001, 320, p 161-176
O. Fabrichnaya, M.J. Kriegel, J. Seidel, G. Savinykh, L.P. Ogorodova, I.A. Kiseleva, and H.J. Seifert, Calorimetric Investigation of the La2Zr2O7, Nd2Zr2O7, Sm2Zr2O7 and LaYO3 Compounds and CALPHAD Assessment of the La2O3-Y2O3 System, Thermochim. Acta, 2011, 526, p 50-57
E.R. Andrievskaya and V.P. Red’ko, Phase Relations in the ZrO2-Y2O3-La2O3 System at 1250°C, Mater. Sci. Forum, 2006, 518, p 343-348
O. Fabrichnaya, G. Savinakh, G. Schreiber, M. Dopita, and H.J. Seifert, Experimental Investigation and Thermodynamic Modelling in the ZrO2-La2O3-Y2O3 System, J. Alloys Compd., 2010, 493, p 263-271
Acknowledgments
Open Access funding provided by Projekt DEAL. S. Sun gratefully acknowledges financial support from CSC. The authors thank Dr. C. Schimpf and B. Bleiber (TU Freiberg) for technical assistance.
Author information
Authors and Affiliations
Corresponding author
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
This invited article is part of a special tribute issue of the Journal of Phase Equilibria and Diffusion dedicated to the memory of Günter Effenberg. The special issue was organized by Andrew Watson, Coventry University, Coventry, United Kingdom; Svitlana Iljenko, MSI, Materials Science International Services GmbH, Stuttgart, Germany; and Rainer Schmid-Fetzer, Clausthal University of Technology, Clausthal-Zellerfield, Germany.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Ilatovskaia, M., Sun, S., Saenko, I. et al. Experimental Investigation of Phase Relations in the ZrO2-La2O3-Yb2O3 System. J. Phase Equilib. Diffus. 41, 311–328 (2020). https://doi.org/10.1007/s11669-020-00790-9
Received:
Revised:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11669-020-00790-9