Abstract
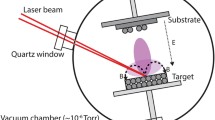
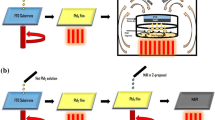
Resonant infrared matrix-assisted pulsed laser evaporation (RIR-MAPLE) was used to deposit the metal-halide perovskite (MHP) CH3NH3PbI3 (methylammonium lead triiodide, or MAPbI), creating phase-pure films. Given the moisture sensitivity of these crystalline, multi-component organic–inorganic hybrid materials, deposition of MAPbI by RIR-MAPLE required a departure from the use of water-based emulsions as deposition targets. Different chemistries were explored to create targets that properly dissolved MAPbI components, were stable under vacuum conditions, and enabled resonant laser energy absorption. Secondary phases and solvent contamination in the resulting films were studied through Fourier transform infrared (FTIR) absorbance and x-ray diffraction (XRD) measurements, suggesting that lingering excess methylammonium iodide (MAI) and low-vapor pressure solvents can distort the microstructure, creating crystalline and amorphous non-perovskite phases. Thermal annealing of films deposited by RIR-MAPLE allowed for excess solvent to be evaporated from films without degrading the MAPbI structure. Further, it was demonstrated that RIR-MAPLE does not require excess MAI to create stoichiometric films with optoelectronic properties, crystal structure, and film morphology comparable to films created using more established spin-coating methods for processing MHPs. This work marks the first time a MAPLE-related technique was used to deposit MHPs.
Similar content being viewed by others
Explore related subjects
Discover the latest articles and news from researchers in related subjects, suggested using machine learning.References
D.B. Mitzi, Progress in Inorganic Chemistry (Hoboken: Wiley, 1999), pp. 1–121.
National Renewable Energy Laboratory. Best Research-Cell Efficiencies. http://www.nrel.gov/ncpv/images/effici ency_chart.jpg. Accessed 25 June 2017.
M.A. Green, A. Ho-Baillie, and H.J. Snaith, Nat. Photonics 8, 506 (2014).
S.D. Stranks, G.E. Eperon, G. Grancini, C. Menelaou, M.J.P. Alcocer, T. Leijtens, L.M. Herz, A. Petrozza, and H.J. Snaith, Science 342, 341 (2013).
W.J. Yin, T. Shi, and Y. Yan, Adv. Mater. 26, 4653 (2014).
G.E. Eperon, S.D. Stranks, C. Menelaou, M.B. Johnston, L.M. Herz, and H.J. Snaith, Energy Environ. Sci. 7, 982 (2014).
A.K. Guria, S.K. Dutta, S. Das Adhikari, and N. Pradhan, ACS Energy Lett. 2, 1014 (2017).
W.A. Dunlap-Shohl, R. Younts, B. Gautam, K. Gundogdu, and D.B. Mitzi, J. Phys. Chem. C 120, 16437 (2016).
S. Shahbazi, C.M. Tsai, S. Narra, C.Y. Wang, H.S. Shiu, S. Afshar, N. Taghavinia, and E.W.G. Diau, J. Phys. Chem. C 121, 3673 (2017).
W. Nie, H. Tsai, R. Asadpour, J.-C. Blancon, A.J. Neukirch, G. Gupta, J.J. Crochet, M. Chhowalla, S. Tretiak, M.A. Alam, H.-L. Wang, and A.D. Mohite, Science 347, 522 (2015).
K. Liang, D.B. Mitzi, and M.T. Prikas, Chem. Mater. 10, 403 (1998).
K. Hwang, Y.S. Jung, Y.J. Heo, F.H. Scholes, S.E. Watkins, J. Subbiah, D.J. Jones, D.Y. Kim, and D. Vak, Adv. Mater. 27, 1241 (2015).
S.-G. Li, K.-J. Jiang, M.-J. Su, X.-P. Cui, J.-H. Huang, Q.-Q. Zhang, X.-Q. Zhou, L.-M. Yang, and Y.-L. Song, J. Mater. Chem. A 3, 9092 (2015).
L.K. Ono, M.R. Leyden, S. Wang, and Y. Qi, J. Mater. Chem. A 4, 6693 (2016).
W.S. Yang, B. Park, E.H. Jung, N.J. Jeon, Y.C. Kim, D.U. Lee, S.S. Shin, J. Seo, E.K. Kim, J.H. Noh, and S. Il Seok, Science 356, 1376 (2017).
M. Liu, M.B. Johnston, and H.J. Snaith, Nature 501, 395 (2013).
D. Yang, Z. Yang, W. Qin, Y. Zhang, S.F. Liu, and C. Li, J. Mater. Chem. A 3, 9401 (2015).
D.B. Mitzi, M.T. Prikas, and K. Chondroudis, Chem. Mater. 11, 542 (1999).
S. Wang, L.K. Ono, M.R. Leyden, Y. Kato, S.R. Raga, M.V. Lee, and Y. Qi, J. Mater. Chem. A 3, 14631 (2015).
W. Ge, T.B. Hoang, M.H. Mikkelsen, and A.D. Stiff-Roberts, Appl. Phys. A Mater. Sci. Process. 122, 824 (2016).
W. Ge, N.K. Li, R.D. McCormick, E. Lichtenberg, Y.G. Yingling, A.D. Stiff-Roberts, and A.C.S. Appl, Mater. Interfaces 8, 19494 (2016).
Q. Yu, W. Ge, A. Atewologun, G.P. López, and A.D. Stiff-Roberts, J. Mater. Chem. B 2, 4371 (2014).
R.D. McCormick, J. Lenhardt, and A.D. Stiff-Roberts, Polymers (Basel) 4, 341 (2012).
W. Ge, A. Atewologun, and A.D. Stiff-Roberts, Org. Electron. 22, 98 (2015).
R. Pate, K.R. Lantz, and A.D. Stiff-Roberts, Thin Solid Films 517, 6798 (2009).
N. Ahn, D.Y. Son, I.H. Jang, S.M. Kang, M. Choi, and N.G. Park, J. Am. Chem. Soc. 137, 8696 (2015).
Y. Guo, K. Shoyama, W. Sato, Y. Matsuo, K. Inoue, K. Harano, C. Liu, H. Tanaka, and E. Nakamura, J. Am. Chem. Soc. 137, 15907 (2015).
L.C. Chen, J.R. Wu, Z.L. Tseng, C.C. Chen, S.H. Chang, J.K. Huang, K.L. Lee, and H.M. Cheng, Materials (Basel). 9, 747 (2016).
L.-C. Chen, C.-C. Chen, J.-C. Chen, and C.-G. Wu, Sol. Energy 122, 1047 (2015).
N.J. Jeon, J.H. Noh, Y.C. Kim, W.S. Yang, S. Ryu, and S. Il Seok, Nat. Mater. 13, 897 (2014).
J.S. Manser, B. Reid, and P.V. Kamat, J. Phys. Chem. C 119, 17065 (2015).
P.P. Khlyabich and Y. Loo, Chem. Mater. 28, 9041 (2016).
Acknowledgements
This work was supported by the National Science Foundation, Research Triangle MRSEC (DMR-1121107). We would like to acknowledge the contributions of Wangyao Ge, Yuankai Liu, and Chenqi Zhao from the Stiff-Roberts group at Duke University in developing the RIR-MAPLE methods described within this work.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflicts of interest.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Barraza, E.T., Dunlap-Shohl, W.A., Mitzi, D.B. et al. Deposition of Methylammonium Lead Triiodide by Resonant Infrared Matrix-Assisted Pulsed Laser Evaporation. J. Electron. Mater. 47, 917–926 (2018). https://doi.org/10.1007/s11664-017-5814-0
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11664-017-5814-0