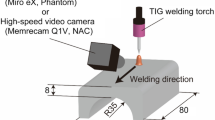
Abstract
It is experimentally shown that a thin layer of silica flux leads to an increased depth of weld penetration during activated TIG (=A-TIG) welding of Armco iron. The oxygen-content is found higher in the solidified weld metal and it is linked to the increased depth of penetration through the reversed Marangoni convection. It is theoretically shown for the first time that the basic reason of the reversed Marangoni convection is the phenomenon called “surface phase transition” (SPT), leading to the formation of a nano-thin FeO layer on the surface of liquid iron. It is shown that the ratio of dissolved oxygen in liquid iron to the O-content of the silica flux is determined by the wettability of silica particles by liquid iron. It is theoretically shown that when the silica flux surface density is higher than 15 µg/mm2, reversed Marangoni flow will take place along more than 50 pct of the melted surface. Comparing the SPT line with the dissociation curves of a number of oxides, they can be positioned in the following order of their ability to serve as a flux for A-TIG welding of steel: anatase-TiO2 (best)-rutile-TiO2 (very good)-silica-SiO2 (good)-alumina-Al2O3 (does not work). Anatase (and partly rutile) are self-regulating fluxes, as they provide at any temperature just as much dissolved oxygen as needed for the reversed Marangoni convection, and not more. On the other hand, oxygen can be over-dosed if silica, and other, less stable oxides (such as iron oxides) are used.






Similar content being viewed by others
References
X. Yuan, M.B. Kim, and C.Y. Kang: Metal Mater Trans A, 2011, vol.42A, pp.1310–1324.
B. Palanisamy and A. Upadhyaya: Metal Mater Trans A, 2011, vol.42A, pp.3417-3424.
G. Zhang, W. Su, J. Zhang, and Z. Wei: Metal Mater Trans A, 2011, vol.42A, pp 2850 – 2861.
X.X. Zhang, B.L. Xiao, and Z.Y. Ma: Metal Mater Trans A, 2011, vol.42A, pp.3218-3228.
X.X. Zhang, B.L. Xiao, and Z.Y. Ma: Metall. Mater. Trans. A, 2011, vol. 42A, pp. 3229–39.
M. Mehta, A. Arora, A. De, and T. DebRoy: Metal Mater Trans A, 2011, vol.42A, pp.2716-2722.
C. Genevois, M. Girard, B. Huneau, X. Sauvage, and G. Racineux: Metal Mater Trans A, 2011, vol.42A, pp.2290-2295.
M. Karadge, B. Grant, P.J. Withers, G. Baxter, and M. Preuss: Metal Mater Trans A, 2011, vol.42A, pp.2301-2311.
K.E. Knipling and R.W. Fonda: Metal Mater Trans A, 2011, vol.42A, pp.2312-2322.
Z. Zhang, B.L. Xiao, D. Wang, and Z.Y. Ma: Metal Mater Trans A, 2011, vol.42A, pp.1717-1726.
D. Bakavos, Y. Chen, L. Babout, P. Prangnell: Metal Mater Trans A, 2011, vol.42A, pp.1266-1282.
E. Cerri, P. Leo, X. Wang, and J.D. Embury: Metal Mater Trans A, 2011, vol.42A, pp.1283-1295.
A. Chamanfar, M. Jahazi, J. Gholipour, P. Wanjara and S. Yue: Metal Mater Trans A, 2011, vol.42A, pp.729-744.
A.L. Pilchak, W. Tang, H. Sahiner, A.P. Reynolds and J.C. Williams: Metal Mater Trans A, 2011, vol.42A, pp.745-762.
K. Chen, W. Gan, K. Okamoto, K. Chung and R.H. Wagoner: Metal Mater Trans A, 2011, vol.42A, pp.488-507.
B. Tam, M.I. Khan and Y. Zhou: Metal Mater Trans A, 2011, vol.42A, pp.2166-2175.
C.W. Chan, H.C. Man and T.M. Yue: Metal Mater Trans A, 2011, vol.42A, pp.2264-2270.
A. Kouadri and L. Barrallier: Metal Mater Trans A, 2011, vol.42A, pp.1815-1826.
S.M. Chowdhury, D.L. Chen, S.D. Bhole, E. Powidajko, D.C. Weckman, and Y. Zhou: Metal Mater Trans A, 2011, vol.42A, pp.1974-1989.
L. Liu and H. Wang: Metal Mater Trans A, 2011, vol.42A, pp.1044-1050.
M.J. Perricone, J.N. DuPont, T.D. Anderson, C.V. Robino and J.R. Michael. Mater Trans A, 2011, vol.42A, pp.700-716.
B.W. Neuberger, P.G. Oberson and S. Ankem: Metal Mater Trans A, 2011, vol.42A, pp.1296-1309.
M. Jeon, J.-H. Lee, T.K. Woo and S. Kim: Metal Mater Trans A, 2011, vol.42A, pp.974-985.
S.M.Gurevich, V.N.Zamkov, N.A.Kushnirenko: Avtom. Svarka, 1965, vol. 9, pp. 1-4.
P.J.Modenesi, E.R.Apolinario, I.M.Pereira: J. Mater. Process. Technol., 2000, vol. 99, pp. 260-265.
S.W.Shyu, H.Y.Huang, K.H.Tseng, C.P.Chou: J. Mater. Eng. Perform., 2008, vol. 17, pp. 193-201.
B.Bonnefois, L.Coudreuse, J.Charles: Welding Intern, 2004, vol.18, pp.208-212.
N. Perry: PhD Thesis, Université Nantes, 2000.
A.G.Simonik: Welding Production, 1976, vol. 3, pp. 49-51.
J.J. Lowke, R.W. Liebermann: J Appl Phys, 1971, vol.43, pp. 1991-1994.
M.M.Savitskii, G.I.Leskov: Avtom Svarka, 1980, vol. 33 (9), pp. 17-22.
T.Sándor, J.Dobránszky: Mater Sci Forum, 2007, vol. 537-538, pp. 63-70.
C.R.Heiple, J.R.Roper: Welding Journal, 1982, vol. 61 (4) pp. 97-102.
P. Nicolas: Thése de doctorat, Universite de Nantes, 2000.
Y.Wang, Q.Shi, H.L.Tsai: Metall. Mater. Trans., 2001, vol. 32B, pp. 145-161.
Y.Wang, H.L.Tsai: Metall Mater Trans, 2001, vol. 32B, pp. 501-14.
Y.P.Lei, H.Murakawa, Y.W.Shi, X.Y.Li: Comp Mater Sci, 2001, vol.21, pp.276-290.
K.C. Mills, E.D. Hondors, Z. Li: J Mater Sci, 2005, vol. 40, pp. 2403-2409.
Y.L.Xu, Z.B.Dong, Y.H.Wei, C.L.Yang: Theor Appl Fracture Mech, 2007, vol.48, pp.178-186.
D.J.Li, S.P.Lu, D.Z.Li, Y.Y.Li: Sci. Technol. Weld. Join., 2010, vol. 15, pp. 528-533.
T-S. Chern, K-H. Tseng, H-L. Tsai: Mater Design, 2011, vol.32, pp.255-263.
T.J.Park, J.P.Kong, S.H.Uhm, I.S.Woo, J.S.Lee, C.Y.Kang: J Mater Process Technol, 2011, vol.211, pp.415-423.
K.-H. Tseng and C.-Y.Hsu: J. Mater. Process. Technol., 2011, vol. 211, pp. 503–12.
D.Li, S.Lu, W.Dong, D.Li, Y.Li: J Mater Process Technol, 2012, vol.212, pp.128-136.
S.Lu, H.Fujii, K.Nogi: Scripta Mater, 2004, vol.51, pp.271-277.
S. Lu, H. Fujii, K. Nogi: Mater. Sci. Eng. A, 2004, vol. 380, pp. 290–97.
S.Lu, H.Fujii, K.Nogi: Metal Mater Trans A, 2004, vol.35A, pp.2861-2866.
S.Lu, H.Fujii, K.Nogi: J Mater Sci, 2005, vol. 40, pp. 2481-2485.
S.Lu, H.Fujii, K.Nogi: J Mater Sci, 2008, vol.43, pp.4583-4591.
H Fuji, T Sato, S Lu and K Nogi: Mater. Sci. Eng. A, 2008, vol.495, pp.296-303.
J.J.Lowke, M.Tanaka, M.Ushio: J. Phys. D., 2005, vol. 38, pp. 3438-3442.
K.A. Yushchenko, D.V. Kovalenko, and I.V. Kovalenko: Paton Weld. J., 2001, No. 7, pp. 37–43.
Y.H. Xiao: PhD thesis, Delft University of Technology, 1992.
A.M.Krouchinin and A.Sawicki: Technical University Czestochowa, Czestochowa, 2003.
G.Kaptay: J. Mater. Sci, 2005, vol. 40, pp. 2125-2131.
G.Kaptay, D.M.Stefanescu: AFS Trans, 1992, vol. 213, pp. 707-712.
J.W.Cahn: J. Chem. Phys., 1977, vol. 66, pp. 3667-3672.
H.Shim, P.Wynblatt, D.Chatain: Surf Sci Lett, 2001, vol. 476, pp. L273-L277.
S.Dogel, D.Nattland, W.Freyland: Thin Solid Films, 2004, vol. 455-456, pp. 380-383.
J.A.V.Butler: Proc. Roy. Soc., 1932, vol. A135, pp. 348-375.
J.Brillo, D.Chatain, I.Egry: Int. J. Mat. Res., 2009, vol. 100, pp. 53-58.
Z.Moser, W.Gasior, J.Pstrus, J. Phase Equilibria, 2001, vol. 22, pp. 254-258.
G. Kaptay: CALPHAD, 2005, vol. 29, pp. 56–67.
C.Mekler, G.Kaptay: Mater. Sci. Eng., 2008, vol. A495, pp. 65-69.
G.Kaptay: J. Disp. Sci. Technol., 2012, vol.33, pp.130-140.
N. Ames and S.Babu: IIW Doc. IX-2195-06.
G.Rückert, B.Huneau, S.Marya: Mater Design, 2007, vol.28, pp. 2387-2393.
T. Sandor and J. Dobranszky: Proc. 6th Eur. Stainless Steel Conf.: Science and Market, P. Karjalainen and S. Hertzman, eds., Helsinki, Jernkontoret, 2008, pp. 837–842.
I. Barin: Thermochemical Properties of Pure Substances, VCh, Weinheim, 1993.
T.B. Massalski: Binary Alloy Phase Diagrams, ASM International, Metals Park, OH, 1990.
D.Bouchard, C.W.Bale: Metall. Mater. Trans, 1995, vol. 26B, pp. 467-484.
J.Miettinen: Calphad, 1998, vol. 22, pp. 231-256.
G.Kaptay: Calphad, 2004, vol. 28, pp. 115-124.
Y.Tang, X.Yuan, Y.Du: JMM B, 2011, vol. 37, pp. 1-10.
C.Guo, C.Li, Z.Du: J. Alloys Compounds, 2009, vol. 492, pp. 122-127.
S.H.Sheng, R.F.Zhang, S.Veprek: Acta Mater, 2011, vol. 59, pp. 3498-3509.
Y.Tang, Y.Du, L.Zhang, X.Yuan, G.Kaptay: Thermochim Acta, 2012, vol.527, pp.131-142.
G.Kaptay: Metall Mater Trans A, 2012, vol.43, pp. 531-543.
P.A.Distin, S.G.Whiteway, C.R.Masson: Canad Met Quart, 1971, vol. 10, pp. 13-18.
T.Iida, R.I.L.Guthrie: ‘The Physical Properties of Liquid Metals’, Clarendon Press, Oxford, 1993.
Slag Atlas, 2nd ed., VDEh Verlag Stahleisen GmbH, Düsseldorf, 1995.
G.Kaptay: Mater. Sci. Eng., 2008, vol. A495, pp. 19-26.
K.Ogino, K.Nogi, C.Hosoi: Tetsu-to-Hagane, 1983, vol. 69, pp. 47-52.
B.J.Keene, K.C.Mills, J.W.Bryant, E.D.Hondros: Canad. Metall. Quart., 1982, vol. 21, pp. 393-403.
H.Taimatsu, K.Nogi, K.Ogino: J. High Temp. Soc., 1992, vol. 18, pp. 14-19.
Z.Jun, K.Mukai: ISIJ International, 1998, vol. 38, pp. 1039-1044.
I.Seyhan, I.Egry: Int. J. Thermophys., 1999, vol. 20, pp. 1017-1028.
D.Mantha, J.P.Hajra: Metall. Mater. Trans, 2001, vol. 32B, pp. 423-427.
W.M. Small, P. Sahoo, K. Li: Scripta Metall. Mater., 1990, vol. 24, pp. 1155–58.
B.Predel: Phase Equilibria, Crystallographic and Thermodynamic Data of Binary Alloys, volume 5 of group IV of Landolt-Börnstein Handbook, Springer, Berlin, pp. 1991–97.
Acknowledgments
The authors acknowledge financial support from the Hungarian Academy of Sciences, under the grant number OTKA K101781. This work was partly financed by TAMOP-4.2.1.B-10/2/KONV-2010-0001 project with support by the European Union and the European Social Fund.
Author information
Authors and Affiliations
Corresponding author
Additional information
Manuscript submitted November 29, 2011.
Appendices
Appendix A: The Thermodynamic Limit of the Dissolved Oxygen Content in Liquid Iron from Silica Dissociation
Let us consider the dissociation of silica in liquid iron according to the following exchange reaction:
where [i] means that component i is dissolved in liquid iron. We consider the equilibrium of almost pure liquid Fe (of unit activity) and pure solid silica (of unit activity) with the liquid iron consisting of the approximately stoichiometric amounts of dissolved Si and FeO. Thus, the stoichiometry of reaction [A1] dictates the following relationship: \( x_{\text{Si}} \cong x_{\text{FeO}} /2 \). Then, the equilibrium constant of Reaction [A1] is written as:
with x i and γ i being the mole fraction and activity coefficient of component i. From standard thermodynamic data the equilibrium constant of Reaction [A1] follows from[69]:
The activity coefficient of Si in infinitely diluted solution of liquid Fe is known from assessed thermodynamic data of[70–72] and is written in the form[73]:
The performance of equation type Eq. [A4] has been proven on different high temperature systems.[74–77] This equation is preferred to other equations at high temperatures as it obeys the 4th law of thermodynamics.[78]
Using data on phase diagram and solubility of the Fe-O system,[70,79] the following approximated equation is obtained in the same formalism as Eq. [A4]:
Substituting Eqs. [A3–A5] into Eq. [A2], the equilibrium solubility of oxygen C O (mass ppm) as a function of temperature is obtained as:
When Eq. [A6] was written, the relationship \( C_{\text{O}} = 2.87 \cdot 10^{5} \cdot x_{\text{FeO}} \) was also taken into account, being valid in diluted solutions of O in almost pure liquid Fe. At the average temperature of the liquid iron of 2260 K (1987 °C) Eq. [A6] provides the value of CO = 2000 ppm, being higher by one order of magnitude compared to our measured values (see Table II: 153 … 203 ppm). Thus, thermodynamic properties of silica do not limit the dissolution of oxygen in liquid iron under the conditions of our experiments at \( \xi > 0.2 \).
Appendix B: The Algorithm to Calculate the SPT Line and Surface Tension of the Liquid Fe-FeO Alloy
Let us consider a binary liquid alloy Fe-FeO alloy at a given temperature (T) and at a given bulk mole fraction of the component FeO (x FeO). According to Butler,[60] the surface of this liquid solution will be in equilibrium with its bulk, if the partial surface tension values of the two components equal:
The partial surface tension value of component i (i = Fe and/or FeO) has been expressed as[60–64]:
where \( \sigma_{i}^{\text{o}} \) is the surface tension of pure component i (J/m2) being only the function of temperature, R is the universal gas constant (8.3145 J/mol K), β is the ratio of surface to bulk coordination numbers of the atoms, ωi is the molar surface area of component i (m2/mol):
where f is a dimensionless geometrical constant, V i is the molar volume of component i (m3/mol), N Av is the Avogadro number, x i and \( x_{i}^{*} \) are the mole fractions of component i in the bulk and in the surface, respectively. The partial excess Gibbs energy of component i (\( \Updelta G_{i}^{\text{E}} \)) is described by the simplest regular solution model:
where Ω is the interaction energy (J/mol), described using Eq. [A5] and the relationship: \( \Upomega = R \cdot T \cdot \ln \gamma_{\text{FeO}}^{\infty } \):
Physical properties of pure liquid Fe and FeO phases needed for calculations are given in Table B1.[80,81] Other model parameters are selected as: β = 9/11, f = 1.00.[82]
In the course of model calculations, first the SPT line for the Fe-FeO system is calculated taking into account the material balance equations in both bulk and surface (\( x_{\text{Fe}} + x_{\text{FeO}} = 1 \) and \( x_{\text{Fe}}^{*} + x_{\text{FeO}}^{*} = 1 \)). At each temperature the bulk x FeO value is searched for, at which the Butler Eq. [B1] has 3 solutions at 3 different FeO surface concentrations, and two of them (at the lowest and highest FeO surface concentrations) provide the same values for surface tension, being somewhat lower compared to the 3rd mathematical solution (for more details see[63,64]). Then, both surface tension and its T-coefficient are calculated as a function of composition and temperature. All calculations are performed numerically, by a home-made software. The calculated surface tension values are in good agreement with the known experimental[83–87] and previously calculated[88,89] values.
Appendix C: Solubility of Oxygen in Liquid Iron Due to the Dissociation of Al2O3
The reaction of dissociation:
The equilibrium constant equals[69]:
The activity coefficient of Al in liquid Fe equals[73,90]:
The equilibrium constant, supposing both Al and O are dissolved via reaction [C1]:
Substituting Eqs. [A5, C2, C3] into Eq. [C4], the equilibrium mole fraction of FeO in liquid iron can be expressed. Using the relationship \( C_{\text{O}} = 2.87 \cdot 10^{5} \cdot x_{\text{FeO}} \), the following final equation is obtained:
Appendix D: Solubility of O in Liquid Iron Due to the Dissociation of TiO2
The reaction of dissociation:
The equilibrium constant for rutile equals[69]:
The activity coefficient of Ti in liquid Fe equals[73,90]:
The equilibrium constant, supposing both Ti and O are dissolved via reaction [D1]:
Substituting Eqs. [A5, D2, D3] into Eq. [D4], the equilibrium mole fraction of FeO in liquid iron can be expressed. Using the relationship \( C_{\rm O} = 2.87 \cdot 10^{5} \cdot x_{\text{FeO}} \), the following final equation is obtained for rutile:
For another form of TiO2 (anatase) the equilibrium constant equals[69]:
Substituting Eqs. [A5, D6, D3] and \( C_{\rm O} = 2.87 \cdot 10^{5} \cdot x_{\text{FeO}} \) into Eq. [D4], the following final equation is obtained for anatase:
Appendix E: Solubility of Oxygen in Liquid Iron Due to Dissociation of Fe2O3
The reaction of dissociation:
The equilibrium constant equals[69]:
The equilibrium constant of reaction [E1]:
Substituting Eqs. [A5, E2] into Eq. [E3], the equilibrium mole fraction of FeO in liquid iron can be expressed. Using the relationship \( C_{\text{O}} = 2.87 \cdot 10^{5} \cdot x_{\text{FeO}} \), the following final equation is obtained:
Rights and permissions
About this article
Cite this article
Sándor, T., Mekler, C., Dobránszky, J. et al. An Improved Theoretical Model for A-TIG Welding Based on Surface Phase Transition and Reversed Marangoni Flow. Metall Mater Trans A 44, 351–361 (2013). https://doi.org/10.1007/s11661-012-1367-2
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11661-012-1367-2