Abstract
Summary
We determined the prospective 10-year association among incident fragility fractures and four glucocorticoid (GC) treatment groups (Never GC, Prior GC, Baseline GC, and Ever GC). Results showed that GC treatment is associated with increased 10-year incident fracture risk in ambulatory men and women across Canada.
Purpose
Using the Canadian Multicentre Osteoporosis Study dataset, we determined the prospective 10-year association between incident fragility fractures and GC treatment.
Methods
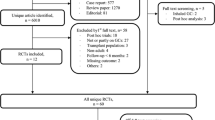
We conducted a 10-year prospective observational cohort study at nine sites across Canada. A total of 9,263 ambulatory men and women 25 years of age and older were included in the analysis. Multivariable Cox proportional hazards analyses were conducted to determine the relationship among GC treatment groups in four levels that included Never GC, Prior GC, Baseline GC, and Ever GC (combined baseline and prior groups) and time to fracture.
Results
In each of the Never GC, Prior GC, Baseline GC, and Ever GC treatment groups, the number of participants were 8,832 (95.4 %), 303 (3.3 %), 128 (1.4 %), and 431 (4.7 %), respectively. Of the 9,263 individuals enrolled, incident fragility non-spine, hip, spine, and any fractures were experienced by a total of 896 (9.67 %), 157 (1.69 %), 130 (1.40 %), and 1,102 (11.90 %) over 10-years, respectively. For men and women combined, prior GC treatment was associated with a higher hazard ratio (HR) for time to incident non-vertebral (HR = 1.5, 95 % confidence interval [CI] = 1.1, 2.0), hip (HR = 2.1, 95 % CI = 1.1, 4.0), and any fracture (HR = 1.4, 95 % CI = 1.0, 1.8) compared with never GC treatment.
Conclusions
GC treatment is associated with increased 10-year incident fracture risk; this highlights the importance of considering therapy to prevent GC-induced fractures for patients who are using GC for various medical conditions.


Similar content being viewed by others
References
Van Staa TP, Leufkens HGM, Abenhaim L, Zhang B, Cooper C (2000) Use of oral corticosteroids and risk of fracture. J Bone Miner Res 15:993–1000
Chavassieux P, Pastoureau P, Chapuy MC, Delmas PD, Meunier PJ (1993) Glucocorticoid-induced inhibition of osteoblastic bone formation in ewes: a biochemical and histomorphometric study. Osteoporos int 2:97–102
Barbarino A, DeMarinis L, Folli G, Tofani A, Della Casa S, D’Amico C et al (1989) Corticotrophin-releasing hormone inhibition of gonadotropin secretion during the menstrual cycle. Metabolism 38:504–506
Van Staa TP, Leufkens HGM, Cooper C (2000) The epidemiology of corticosteroid-induced osteoporosis: a meta-analysis. Osteoporos Int 13:777–787
Van Staa TP, Leufkens HG, Abenhaim L, Zhang B, Cooper C (2000) Oral corticosteroids and fracture risk: relationship to daily and cumulative doses. Rheumatology (Oxford) 39(12):1383–1389
Papaioannou A, Morin S, Cheung A, Atkinson S, Brown JP, Feldman S et al (2010) Clinical practice guidelines for the diagnosis and management of osteoporosis in Canada: summary. CMAJ 182:1864–1872
Kanis, Burlet N, On behalf of the European Society for Clinical and Economic Aspects of Osteoporosis and Osteoarthritis (ESCEO) (2008) European guidance for the diagnosis and management of osteoporosis in postmenopausal women. Osteoporos Int 19:399–428
National Osteoporosis Foundation (2008) Clinician’s guide to prevention and treatment of osteoporosis. Washington, DC: National Osteoporosis Foundation. www.nof.org. Accessed September 2013
Kanis JA on behalf of the World Health Organization Scientific Group (2008) assessment of osteoporosis at the primary health-care level. Technical report. Who collaborating Centre, University of Sheffield, UK. Accessed at www.shef.ac.uk/FRAX/. Accessed September 2013
Kanis JA, Johnell O, Oden A, Johansson H, McCloskey E (2008) FRAX and the assessment of fracture probability in men and women from the UK. Osteoporos Int 19:385–397
Siminoski K, Leslie W, Frame H, Hodsman A, Josse RG, Khan A et al (2005) Recommendations for bone mineral density reporting in Canada. Can Assoc Radiol J 56:178–188
Leslie WD, Berger C, Langsetmo L, Lix LM, Adachi JD, Hanley G et al (2011) Construction and validation of a simplified fracture risk assessment tool for Canadian women and men: from the CaMos and Manitoba cohorts. Osteoporos Int 6:1873–1883
Kreiger N, Tenenhouse A, Joseph L, Mackenzie T, Poliquin S, Brown JP et al (1999) The Canadian Multicentre Osteoporosis Study (CaMos): background, rationale, methods. Can J Aging 18:376–387
Jackson SA, Tenenhouse A, Robertson L, CaMos Study Group (2000) Vertebral fracture definition from population-based data: preliminary results from the Canadian Multicenter Osteoporosis Study (CaMos). Osteoporos Int 8:680–687
Tarride JE, Hopkins RB, Leslie WE, Morin S, Adachi JD, Papaioannou A et al (2012) The burden of illness of osteoporosis in Canada. Osteoporos Int 23:2591–25600
Adachi JD, Ioannidis G, Berge C, Joseph L, Papaioannou A, Pickard L et al (2001) The influence of osteoporotic fractures on Health related quality of life in community dwelling men and women across Canada. Osteoporos Int 12:903–908
Ioannidis G, Papaioannou A, Hopman WM, Akhtar-Danesh N, Anastassiades T, Pichard L et al (2009) Relation between fractures and mortality: results from the Canadian multicentre osteoporosis study. CMAJ 181:265–271
Kanis JA, Johansson H, Oden A, Johnell O, de Laet C, Melton LJ III et al (2004) A meta-analysis of prior corticosteroid use and fracture risk. J Bone Miner Res 19:893–899
Kanis JA, Johansson H, Oden A, McCloskey M (2011) Guidance for the adjustment of FRAX according to the dose of glucocorticoids. Osteoporos Int 22:809–816
Steinbuch M, Youket TE, Cohen S (2004) Oral glucocorticoid use is associated with an increased risk of fracture. Osteoporos Int 15:323–328
Van Staa TP, Leufkens HG, Cooper C (2002) The epidemiology of corticosteroid-induced osteoporosis: a meta-analysis. Osteoporos Int 13:777–787
Salto JK, Davis JW, Wasnich RD, Ross PD (1995) Users of low-dose glucocorticoids have increases bone loss rates: a longitudinal study. Calcif Tissue Int 57:115–119
Adachi JD, Bensen WG, Bell MJ et al (1990) Corticosteroid induced osteoporosis: follow-up over 3 years. In: Christiansen C, Overgaard K (eds) Osteoporosis 1990 (3). Third International Symposium on Osteoporosis, Copenhagen, pp 1745–1747
Natsui K, Tanaka K, Suda M, Yasoda A, Sakuma Y, Ozasa A et al (2006) High-dose glucocorticoid treatment induces rapid loss of trabecular bone mineral density and lean body mass. Osteoporos Int 17:105–108
Gonnelli S, Caffarelli C, Maggi S, Guglielmi G, Siviero P, Rossi S et al (2010) EOLO study group. Effect of inhaled glucocorticoids and beta(2) agonists on vertebral fracture risk in COPD patients: the EOLO study. Calcif Tissue Int 87:137–147
Vestergaard P, Rejnmark L, Mosekilde L (2008) Fracture risk associated with different types of oral corticosteroids and effect of termination of corticosteroids on the risk of fractures. Calcif Tissue Int 82:249–257
Cadarette SM, Jaglal SB, Raman-Wilms L, Beaton DE, Paterson JM (2011) Osteoporosis quality indicators using healthcare utilization data. Osteoporos Int 22:1335–1342
Chen Z, Kooperberg C, Pettinger MB, Bassford T, Cauley JA, LaCroix AZ et al (2004) Validity of self-report for fractures among a multiethnic cohort of postmenopausal women: results from the Women’s Health Initiative observational study and clinical trials. Menopause 11(3):264–274
Conflicts of interest
G. Ioannidis, S. Pallan, M. Mulgund, L. Rios, J. Ma, L. Thabane, K. S. Davison, C. S. Kovacs, N. Kreiger, J. C. Prior, and T. Towheed declare that they have no conflict of interest.
A. Papaioannou: Speaker's bureau: Amgen, Eli Lilly, Merck, Novartis, Procter & Gamble, Sanofi Aventis, Warner Chilcott. Research grants: Amgen, Eli Lilly, Merck, Novartis, Procter & Gamble, Sanofi Aventis, Warner Chilcott. Consulting fees or other remuneration (payment): Amgen, Eli Lilly, Merck, Novartis, Procter & Gamble, Roche, Sanofi Aventis, Warner Chilcott, Wyeth.
R. G. Josse: Advisory board member: Amgen, Lilly, Merck, Novartis, BMS/AZ, Janssen. Speaker honoraria: Amgen, Lilly, Merck, Novartis, BMS/AZ, Janssen. Research grants: Amgen, Janssen, BMS/AZ.
W. P. Olszynski: Consultant or on a speaker's bureau for Amgen, Eli Lilly, Merck Frosst Canada, Novartis, Pfizer, Procter & Gamble Pharmaceuticals and sanofi-aventis.
J. D. Adachi: Speaker's bureau: Amgen, Eli Lilly, Merck, Novartis, Procter & Gamble, Roche, Sanofi Aventis, Warner Chilcott. Research grants: Amgen, Bristol-Myers Squibb, Eli Lilly, Merck, Novartis, Pfizer, Procter & Gamble, Roche, Sanofi Aventis, Warner Chilcott, Wyeth. Consulting fees or other remuneration (payment): Amgen, Eli Lilly, GSK, Merck, Novartis, Pfizer, Procter & Gamble, Roche, Sanofi Aventis, Warner Chilcott, Wyeth.
Grant support
The Canadian Multicentre Osteoporosis Study was funded by the Canadian Institutes of Health Research (CIHR), Amgen, Merck Frosst Canada Ltd., Eli Lilly Canada Inc., Novartis Pharmaceuticals Inc., The Alliance: sanofi-aventis and Procter and Gamble Pharmaceuticals Canada Inc., The Dairy Farmers of Canada, and The Arthritis Society.
Author information
Authors and Affiliations
Consortia
Corresponding author
Rights and permissions
About this article
Cite this article
Ioannidis, G., Pallan, S., Papaioannou, A. et al. Glucocorticoids predict 10-year fragility fracture risk in a population-based ambulatory cohort of men and women: Canadian Multicentre Osteoporosis Study (CaMos). Arch Osteoporos 9, 169 (2014). https://doi.org/10.1007/s11657-013-0169-5
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s11657-013-0169-5