Abstract
Objective
To investigate the effects of potassium alum (Alunite) on smooth muscle contraction and phosphorylation of myosin light chain by myosin light chain kinase (MLCK) and to try to find out the clue of its mechanism.
Methods
An isolated rabbit duodenum smooth muscle strip was selected to study the effects of potassium alum on its contractile activity under the condition of Krebs’ solution using HW-400S constant temperature smooth muscle trough. The myosin and MLCK were purified from chicken gizzard smooth muscle. Myosin light chain phosphorylation was determined by glycerol-polyacrylamide gel electrophoresis; myosin Mg2+-ATPase activity was measured by inorganic phosphate liberation method.
Results
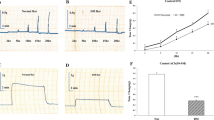
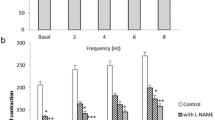
Potassium alum (2.5–20 mmol/L) inhibited the contraction on duodenum in a dose-related and a time-dependent manner; potassium alum could also inhibit the extent of phosphorylation of myosin light chain in a dose-related and a time-dependent manner; and potassium alum inhibited the extent of Mg2+-ATPase activity in a dose-related manner.
Conclusions
Potassium alum inhibited smooth muscle contraction in a way of inhibiting phosphorylation of myosin light chain and Mg2+-ATPase activity. This has revealed the molecular mechanism of treatment of gastrointestinal spastic disorders by potassium alum.
Similar content being viewed by others
References
Dutta S, De SP, Bhattacharya SK. In vitro antimicrobial activity of potash alum. Ind J Med Res 1996;104:157–159.
Luo DQ, Zhao YK, Zhang WJ, Wu LC. Aquagenic acrokeratoderma. Int J Dermatol 2010;49:526–531.
Sugawara C, Sugawara N, Kiyosawa H, Miyake H. Decrease of serum triglyceride in normal rat fed with 2000 ppm aluminum diet for 67 days. II. Feeding young and adult rats a sucrose diet with addition of aluminum hydroxide and aluminum potassium sulfate. Fundam Appl Toxicol 1988;10:616–623.
Udom EJ, Umoh MS, Udosen EO. Recto-vaginal fistula following coitus: an aftermath of vaginal douching with aluminium potassium sulphate dodecahydrate (potassium alum). Int J Gynaecol Obstet 1999;66:299–300.
Hinke SA. Diamyd, an alum-formulated recombinant human GAD65 for the prevention of autoimmune diabetes. Curr Opin Mol Ther 2008;10:516–525.
Kim SH, Park SA, Kim HK, Cho YJ, Kim KS, Kim YH, et al. Enhancement of the immune responses of mice to Bacillus anthracis protective antigen by CIA07 combined with alum. Arch Pharm Res 2008;31:1385–1392.
Falsey AR, Walsh EE, Capellan J, Gravenstein S, Zambon M, Yau E, et al. Comparison of the safety and immunogenicity of 2 respiratory syncytial virus (RSV) vaccines—nonadjuvanted vaccine or vaccine adjuvanted with alum given concomitantly with influenza vaccine to high-risk elderly individuals. J Infect Dis 2008;198:1317–1326.
Liang Y, Ma S, Yang Z, Liu L, Wang L, Wang J, et al. Immunogenicity and safety of a novel formalin-inactivated and alum-adjuvanted candidate subunit vaccine for mumps. Vaccine 2008;26:4276–4283.
Inoue K, Takano H, Koike E, Warabi E, Yanagawa T, Yanagisawa R, et al. Peroxiredoxin I is a negative regulator of Th2-dominant allergic asthma. Int Immunopharmacol 2009;9:1281–1288.
Kool M, Pétrilli V, De Smedt T, Rolaz A, Hammad H, van Nimwegen M, et al. Cutting edge: alum adjuvant stimulates inflammatory dendritic cells through activation of the NALP3 inflammasome. J Immunol 2008;181:3755–3759.
De Gregorio E, Tritto E, Rappuoli R. Alum adjuvanticity: unraveling a century old mystery. Eur J Immunol 2008;38:2085–2089.
Li H, Willingham SB, Ting JP, Re F. Cutting edge: inflammasome activation by alum and alum’s adjuvant effect are mediated by NLRP3. J Immunol 2008;181:17–21.
Serre K, Mohr E, Toellner KM, Cunningham AF, Granjeaud S, Bird R, et al. Molecular differences between the divergent responses of ovalbumin-specific CD4 T cells to alumprecipitated ovalbumin compared to ovalbumin expressed by Salmonella. Mol Immunol 2008;45:3558–3566.
Hachiro Y, Kunimoto M, Abe T, Kitada M, Ebisawa Y. Aluminum potassium sulfate and tannic acid injection in the treatment of total rectal prolapse: early outcomes. Dis Colon Rectum 2007;50:1996–2000.
Tokunaga Y, Sasaki H, Saito T. Evaluation of sclerotherapy with a new sclerosing agent and stapled hemorrhoidopexy for prolapsing internal hemorrhoids: retrospective comparison with hemorrhoidectomy. Dig Surg 2010;27:469–472.
Singh HP, Singh CK, Singh RR. Effect of potash alum (aluminium potassium sulphate) on human semen and sperm. Indian J Physiol Pharmacol 1998;42:311–314.
Zámbó L, Dékány M, Bender T. The efficacy of alumcontaining ferrous thermal water in the management of chronic inflammatory gynaecological disorders-a randomized controlled study. Eur J Obstet Gynecol Reprod Biol 2008;140:252–257.
Sharona Even-Ram, Andrew D. Doyle, Mary Anne Conti, Kazue Matsumoto, Robert S. Adelstein, Kenneth M. Yamada. Myosin IIA regulates cell motility and actomyosin-microtubule crosstalk. Nature Cell Biol 2007;9:299–309.
Oneda S, Takasaki T, Kuriwaki K, Ohi Y, Umekita Y, Hatanaka S, et al. Chronic toxicity and tumorigenicity study of aluminum potassium sulfate in B6C3F1 mice. In Vivo 1994;8:271–278.
Kawasaki Y, Umemura T, Sai K, Hasegawa R, Momma J, Saitoh M, et al. A 13-week toxicity study of simultaneous administration of cochineal and aluminum potassium sulfate in rats. Eisei Shikenjo Hokoku 1994;112:48–56.
Ozturk Y, Yildizoglu-Ari N, Altan N, Altan VM. Effect of potassium alumn on gastro-intestinal complications in alloxan-diabetic rats. Gen Pharmacol 1992;23:769–773.
Wu YH, Zhou ZM, Xiong YL, Wang YL, Sun JH, Liao HB, et al. Effects of aluminum potassium sulfate on learning, memory, and cholinergic system in mice. Acta Pharmacol Sin 1998;19:509–512.
Zhang WQ, Xu GS, Huang GW. Chronic toxic effects of aluminum on nervous system in rabbits. Chin J Prevent Med (Chin) 1994;28:158–161.
Chih-Hung Guoa, Guoo-Shyng W. Hsub, Chia-Ju Chuanga, Pei-Chung Chena. Aluminum accumulation induced testicular oxidative stress and altered selenium metabolism in mice. Environment Toxicol Pharmacol 2009;27:176–181.
Tang Z, Chen H, Yang J, DAI S, LIN Y. The comparison of Ca2+/CaM-independent and Ca2+/CaM-dependent phosphorylation of myosin light chains by MLCK. Physiol Res 2005;54:671–678.
Schümann K, Hunder G. A modified device for the differentiated study of intestinal transfer in isolated intestinal segments from mice and suckling rats in vitro. J Pharmacol Toxicol Methods 1996;36:211–217.
Kodama T, Fukui K, Kometani K. The initial phosphate burst in ATP hydrolysis by myosin and subfragment-1 as studied by a modified malachite green method for determination of inorganic phosphate. J Biochem (Tokyo) 1986;99:1465–1472.
Bradford MM. A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal Biochem 1976;7:248–254.
Tansey MG, Luby-Phelps K, Kamm KE, Stull JT. Ca2+-dependent phosphorylation of myosin light chain kinase decreases the Ca2+ sensitivity of light chain phosphorylation within smooth muscle cells. J Biol Chem 1994;269:9912–9920.
Herring BP, Dixon S, Gallagher PJ. Smooth muscle myosin light chain kinase expression in cardiac and skeletal muscle. Am J Physiol Cell Physiol 2000;279:C1656–C1664.
Hodge T, Cope MJ. A myosin family tree. J Cell Sci 2000;113:3353–3354.
Rayment I, Holden HM, Whittaker M, Yohn CB, Lorenz M, Holmes KC, et al. Structure of the actin-myosin complex and its implications for muscle contraction. Science 1993;261:58–65.
Takano-Ohmuro H, Kohama K. Analysis of smooth muscle myosin phosphorylation with native pyrophosphate gels and its application to studies on myosin phosphorylation in contracting smooth muscle. J Biochem 1986;100:1681–1684.
Uehara R, Hosoya H, Mabuchi I. In vivo phosphorylation of regulatory light chain of myosin II in sea urchin eggs and its role in controlling myosin localization and function during cytokinesis. Cell Motil Cytoskeleton 2008;65:100–115.
Thomas DD, Ramachandran S, Roopnarine O, Hayden DW, Ostap EM. The mechanism of force generation in myosin: a disorder-to-order transition, coupled to internal structural changes. Biophys J 1995;68:135S–141S.
Author information
Authors and Affiliations
Corresponding author
Additional information
Supported by the National Natural Science Foundation of China (No. 30772601)
Postgraduate student
Rights and permissions
About this article
Cite this article
Tang, Zy., Lin, Y., Yang, Xl. et al. Inhibitory effect of potassium alum on smooth muscle contraction of rabbit and its mechanism. Chin. J. Integr. Med. (2014). https://doi.org/10.1007/s11655-014-1330-5
Received:
Published:
DOI: https://doi.org/10.1007/s11655-014-1330-5