Abstract
Objective
To extract tumor interstitial fluid (TIF) from MKN-45 gastric cancer which is similar to “muddy phlegm” in Chinese medicine and observe influences of MKN-45 tumor interstitial fluid (MKN-45 TIF) intervention on metastasis of gastric cancer and on the expressions of vascular endothelial growth factor (VEGF), kinase insert domain containing receptor (KDR), epithelial-cadherin (E-cad), cyclooxygenase-2 (COX-2), intercellular adhesion molecule-1 (ICAM-1) and telomerase genes and proteins in primary tumor tissue.
Methods
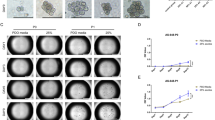
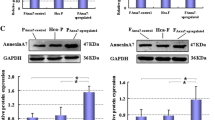
An MKN-45 tumor-bearing model was established in 50 nude mice. The modeled animals were equally randomized to 5 groups: the simple tumor-bearing group (model group), the normal saline (NS) via tail vein injection (i.v.) group (NS i.v. group), MKN-45 TIF i.v. group (TIF i.v. group), NS intraperitoneal injection (i.p.) group (NS i.p. group), and MKN-45 TIF i.p. group (TIF i.p. group). The TIF and NS intervention groups received injection (i.p. or i.v.) of MKN-45 TIF or NS twice a week, 0.2 mL at a time. After 8 weeks, the primary tumors were removed, weighed and HE stained to observe tumor metastasis. The primary tumor tissues were analyzed by immunohistochemistry and real-time quantitative PCR to detect expressions of VEGF, KDR, E-cad, COX-2, ICAM-1, and telomerase genes and proteins in different groups.
Results
There were significant differences in tumor weight between TIF intervention groups and the model and NS intervention groups. Tumor metastasis was observed in all 5 groups, but the tumor metastasis rate in TIF intervention groups was significantly higher than those in the model and NS intervention groups. The gene and protein expressions of gastric cancer-related factors VEGF, KDR, COX-2, ICAM-1 and telomerase were unregulated while the gene and protein expressions of E-cad were downregulated in TIF intervention groups.
Conclusions
TIF promotes tumor growth, invasion and metastasis of gastric cancer. These findings provide preliminary experimental clues for verifying the hypothesis of “tumor-phlegm microenvironment”.
Similar content being viewed by others
References
Böhner H, Zimmer T, Hopfenmüller W, Berger G, Buhr HJ. Detection and prognosis of recurrent gastric cancer—is routine follow-up after gastrectomy worthwhile? Hepatogastroenterology 2000;47:1489–1494.
Sun DZ, Ju DW, He J, Lu Y, Wu F, Li C, et al. Tumor interstitial fluid and postoperative recurrence of tumors: an experimental study for verifying hypothesis of “tumor-phlegm microenvironment”. Chin J Integr Med 2010;16:435–441.
Sun DZ, Xu L, Wei PK, Liu L, He J. Syndrome differentiation in traditional Chinese medicine and E-cadherin/ICAM-1 gene protein expression in gastric carcinoma. World J Gastroenterol 2007;13:4321–4327.
Liao YP, Schaue D, McBride WH. Modification of the tumor microenvironment to enhance immunity. Front Biosci 2007;12:3576–3600.
Sautès-Fridman C, Cherfils-Vicini J, Damotte D, Fisson S, Fridman WH, Cremer I, et al. Tumor microenvironment is multifaceted. Cancer Metastasis Rev 2011;30:13–25.
Lee SA, Park KH, Lee JW. Modulation of signaling between TM4SF5 and integrins in tumor microenvironment. Front Biosci 2011;16:1752–1758.
Chouaib S, Kieda C, Benlalam H, Noman MZ, Mami-Chouaib F, Rüegg C. Endothelial cells as key determinants of the tumor microenvironment: interaction with tumor cells, extracellular matrix and immune killer cells. Crit Rev Immunol 2010;30:529–545.
Eccles SA. Metastasis and the tumor microenvironment: a joint metastasis research society—AACR conferenceresearch on metastasis: Part 1. IDrugs 2010;13:765–767.
Pardee AD, McCurry D, Alber S, Hu P, Epstein AL, Storkus WJ. A therapeutic OX40 agonist dynamically alters dendritic, endothelial, and T cell subsets within the established tumor microenvironment. Cancer Res 2010;70:9041–9052.
Rakhra K, Bachireddy P, Zabuawala T, Zeiser R, Xu L, Kopelman A, et al. CD4+ T cells contribute to the remodeling of the microenvironment required for sustained tumor regression upon oncogene inactivation. Cancer Cell 2010;18:485–498.
Rofstad EK, Ruud EB, Mathiesen B, Galappathi K. Associations between radiocurability and interstitial fluid pressure in human tumor xenografts without hypoxic tissue. Clin Cancer Res 2010;16:936–945.
Moen I, Tronstad KJ, Kolmannskog O, Salvesen GS, Reed RK, Stuhr LE. Hyperoxia increases the uptake of 5-fluorouracil in mammary tumors independently of changes in interstitial fluid pressure and tumor stroma. BMC Cancer 2009;9:446.
Rofstad EK, Gaustad JV, Brurberg KG, Mathiesen B, Galappathi K, Simonsen TG. Radiocurability is associated with interstitial fluid pressure in human tumor xenografts. Neoplasia 2009;11:1243–1251.
Haider MA, Sitartchouk I, Roberts TP, Fyles A, Hashmi AT, Milosevic M. Correlations between dynamic contrast-enhanced magnetic resonance imaging-derived measures of tumor microvasculature and interstitial fluid pressure in patients with cervical cancer. J Magn Reson Imag 2007;25:153–159.
Smith JH, Humphrey JA. Interstitial transport and transvascular fluid exchange during infusion into brain and tumor tissue. Microvasc Res 2007;73:58–73.
Ferretti S, Allegrini PR, Becquet MM, McSheehy PM. Tumor interstitial fluid pressure as an early-response marker for anticancer therapeutics. Neoplasia 2009;11:874–881.
Augsten M, Hägglöf C, Peña C, Ostman A. A digest on the role of the tumor microenvironment in gastrointestinal cancers. Cancer Microenviron 2010;3:167–176.
Hofmann M, Guschel M, Bernd A, Bereiter-Hahn J, Kaufmann R, Tandi C, et al. Lowering of tumor interstitial fluid pressure reduces tumor cell proliferation in a xenograft tumor model. Neoplasia 2006;8:89–95.
Lunt SJ, Chaudary N, Hill RP. The tumor microenvironment and metastatic disease. Clin Exp Metastasis 2009;26:19–34.
Onimaru M, Yonemitsu Y. Angiogenic and lymphangiogenic cascades in the tumor microenvironment. Front Biosci (Schol Ed) 2011;3:216–225.
Kuperwasser C. The tumor stromal microenvironment as modulator of malignant behavior. J Mammary Gland Biol Neoplasia. 2010;15:377–379.
Cook LM, Hurst DR, Welch DR. Metastasis suppressors and the tumor microenvironment. Semin Cancer Biol 2011;21:113–122.
Eccles SA. Metastasis and the tumor microenvironment: a joint metastasis research society-AACR conference research on metastasis: part 2. IDrugs 2010;13:768–771.
Heidenreich R, Machein M, Nicolaus A, Hilbig A, Wild C, Clauss M, et al. Breier G. Inhibition of solid tumor growth by gene transfer of VEGF receptor-1 mutants. Int J Cancer 2004;111:348–357.
Ding S, Li C, Lin S, Han Y, Yang Y, Zhang Y, et al. Distinct roles of VEGF-A and VEGF-C in tumour metastasis of gastric carcinoma. Oncol Rep 2007;17:369–375.
Zheng H, Takahashi H, Murai Y, Cui Z, Nomoto K, Niwa H, et al. Expressions of MMP-2, MMP-9 and VEGF are closely linked to growth, invasion, metastasis and angiogenesis of gastric carcinoma. Anticancer Res 2006;26:3579–3583.
Ishikawa M, Kitayama J, Kazama S, Nagawa H. Expression of vascular endothelial growth factor C and D (VEGF-C and -D) is an important risk factor for lymphatic metastasis in undifferentiated early gastric carcinoma. Jpn J Clin Oncol 2003;33:21–27.
Zhao A, Dou K, Li K, Fu Y. The effects of the expression of VEGF and KDR on the angiogenesis, growth and metastasis of hepatocellular carcinoma. Chin J Surg (Chin) 2000;38:453–456.
Takahashi Y, Kitadai Y, Bucana CD, Cleary KR, Ellis LM. Expression of vascular endothelial growth factor and its receptor, KDR, correlates with vascularity, metastasis, and proliferation of human colon cancer. Cancer Res 1995;55:3964–3968.
Shiozaki H, Oka H, Inoue M, Tamura S, Monden M. E-cadherin mediated adhesion system in cancer cells. Cancer 1996;77:1605–1613.
Kim DY, Joo JK, Park YK, Ryu SY, Kim HS, Noh BK, et al. E-cadherin expression in early gastric carcinoma and correlation with lymph node metastasis. J Surg Oncol 2007;96:429–435.
van Velthuysen ML, Taal BG, van der Hoeven JJ, Peterse JL. Expression of oestrogen receptor and loss of E-cadherin are diagnostic for gastric metastasis of breast carcinoma. Histopathology 2005;46:153–157.
Wang CX, Chen J, Yang Z, Song W, Du ZL, Li ZH. Correlation of E-cadherin, beta-catenin and gamma-catenin gene expression with the invasion and metastasis of gastric carcinoma. J First Milit Med Univ (Chin) 2003;23:1198–1201.
Li Z, Schem C, Shi YH, Medina D, Zhang M. Increased COX2 expression enhances tumor-induced osteoclastic lesions in breast cancer bone metastasis. Clin Exp Metastasis 2008;25:389–400.
Mutoh H, Hayakawa H, Sakamoto H, Sugano K. Homeobox protein CDX2 reduces Cox-2 transcription by inactivating the DNA-binding capacity of nuclear factor-kappaB. J Gastroenterol 2007;42:719–729.
Gross I, Duluc I, Benameur T, Calon A, Martin E, Brabletz T, et al. The intestine-specific homeobox gene Cdx2 decreases mobility and antagonizes dissemination of colon cancer cells. Oncogene 2008;27:107–115.
Wang BC, Guo CQ, Sun C, Sun QL, Liu GY, Li DG. Mechanism and clinical significance of cyclooxygenase-2 expression in gastric cancer. World J Gastroenterol 2005;11:3240–3244.
Koyama S, Ebihara T, Fukao K. Expression of intercellular adhesion molecule 1 (ICAM-1) during the development of invasion and/or metastasis of gastric carcinoma. J Cancer Res Clin Oncol 1992;118:609–614.
Aoudjit F, Potworowski EF, Springer TA, St-Pierre Y. Protection from lymphoma cell metastasis in ICAM-1 mutant mice: a posthoming event. J Immunol 1998;161:2333–2338.
Lalancette M, Aoudjit F, Potworowski EF, St-Pierre Y. Resistance of ICAM-1-deficient mice to metastasis overcome by increased aggressiveness of lymphoma cells. Blood 2000;95:314–319.
Sunami T, Yashiro M, Chung KH. ICAM-1 (intercellular adhesion molecule-1) gene transfection inhibits lymph node metastasis by human gastric cancer cells. Jpn J Cancer Res 2000;91:925–933.
Izumi K, Ishikawa K, Tojigamori M, Matsui Y, Shiraishi N, Kitano S. Liver metastasis and ICAM-1 mRNA expression in the liver after carbon dioxide pneumoperitoneum in a murine model. Surg Endosc 2005;19:1049–1054.
Dikmen ZG, Ozgurtas T, Gryaznov SM, Herbert BS. Targeting critical steps of cancer metastasis and recurrence using telomerase template antagonists. Biochim Biophys Acta 2009;1792:240–247.
Kishimoto H, Kojima T, Watanabe Y, Kagawa S, Fujiwara T, Uno F, et al. In vivo imaging of lymph node metastasis with telomerase-specific replication-selective adenovirus. Nat Med 2006;12:1213–1219.
Kojima T, Watanabe Y, Hashimoto Y, Kuroda S, Yamasaki Y, Yano S, et al. In vivo biological purging for lymph node metastasis of human colorectal cancer by telomerase-specific oncolytic virotherapy. Ann Surg 2010;251:1079–1086.
Author information
Authors and Affiliations
Corresponding authors
Additional information
Supported by the Major Research Program Grant of the National Science Foundation (No. 90709044), and China Postdoctoral Science Foundation Project (No. 20100480096)
Rights and permissions
About this article
Cite this article
Sun, Dz., Jiao, Jp., Ju, Dw. et al. Tumor interstitial fluid and gastric cancer metastasis: An experimental study to verify the hypothesis of “tumor-phlegm microenvironment”. Chin. J. Integr. Med. 18, 350–358 (2012). https://doi.org/10.1007/s11655-012-1085-z
Received:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11655-012-1085-z