Abstract
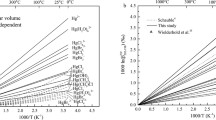
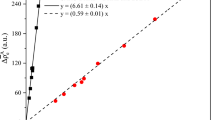
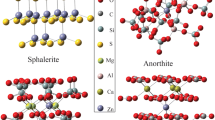
To investigate equilibrium mercury (Hg) and lead (Pb) isotope fractionation caused by the nuclear volume effect (NVE) in crystals, the electron densities at nuclei (i.e., |Ψ(0)|2) for Hg- or Pb-bearing crystalline compounds were investigated by using the relativistic spin orbit zeroth-order regular approximation (ZORA) method with a three-dimensional periodic boundary condition based on the density functional theory (DFT). Many isotope fractionation factors of crystalline compounds are provided for the first time. Our results show, even at 1000 °C, NVE-driven Hg and Pb isotope fractionation are meaningfully large, i.e., range from 0.12‰ to 0.49‰ (202Hg/198Hg), from − 0.20‰ to 0.17‰ (208Pb/206Pb) and from − 0.08‰ to 0.06‰ (207Pb/206Pb) relative to Hg0 vapor and Pb0 vapor, respectively. Specifically, the fractionations range from − 0.06‰ to − 0.20‰ (208Pb/206Pb) and from − 0.02‰ to − 0.08‰ (207Pb/206Pb) for Pb2+-bearing species, from 0.10‰ to 0.17‰ (208Pb/206Pb) and from 0.04‰ to 0.06‰ (207Pb/206Pb) for Pb4+-bearing species in crystals. All calculated Hg-bearing species in crystals will enrich heavier isotope (202Hg) relative to Hg0 vapor. Meanwhile, Pb4+-bearing species enrich heavier Pb isotopes (208Pb and 207Pb) than Pb2+-bearing species in crystals, which the enrichment can be up to 0.37‰ (208Pb/206Pb) and 0.14‰ (207Pb/206Pb) at 1000 °C, due to their NVEs are in opposite directions. The NVE-driven MIFs of Hg isotopes, which are compared to the Hg202- Hg198 baseline, are up to − 0.158‰ (\(\Delta {}_{{{\text{NV}}}}^{199} {\text{Hg}}\)), − 0.024‰ (\(\Delta {}_{{{\text{NV}}}}^{200} {\text{Hg}}\)) and − 0.094‰ (\(\Delta {}_{{{\text{NV}}}}^{201} {\text{Hg}}\)) relative to Hg0 vapor at 500 °C. For all studied Hg-bearing species in crystals, the MIFs of two odd-mass isotopes (i.e., \(\Delta {}_{{{\text{NV}}}}^{199} {\text{Hg}}\) and \(\Delta {}_{{{\text{NV}}}}^{201} {\text{Hg}}\)) will be changed proportionally and their ratio (i.e., \(\Delta {}_{{{\text{NV}}}}^{199} {\text{Hg}}\)/\(\Delta {}_{{{\text{NV}}}}^{201} {\text{Hg}}\)) will be a constant 1.67. The NVE can also cause mass-independent fractionations for 207Pb and 204Pb compared to the baseline of 208Pb and 206Pb. The largest NVE-driven MIFs are 0.043‰ (\(\Delta {}_{{{\text{NV}}}}^{207} {\text{Pb}}\)) and − 0.040‰ (\(\Delta {}_{{{\text{NV}}}}^{204} {\text{Pb}}\)) among all the studied species relative to Pb0 vapor at 500 °C. The magnitudes of odd-mass isotope MIF (\(\Delta {}_{{{\text{NV}}}}^{207} {\text{Pb}}\)) and even-mass isotope MIF (\(\Delta {}_{{{\text{NV}}}}^{204} {\text{Pb}}\)) are almost the same but with opposite signs, leading to the MIF ratio of them (i.e., \(\Delta {}_{{{\text{NV}}}}^{207} {\text{Pb}}\)/\(\Delta {}_{{{\text{NV}}}}^{204} {\text{Pb}}\)) is − 1.08.





Similar content being viewed by others
References
Abe M, Suzuki T, Fujii Y, Hada M, Hirao K (2008) An ab initio molecular orbital study of the nuclear volume effects in uranium isotope fractionations. J Chem Phys 129:164309-1-164309–7
Abe M, Suzuki T, Fujii Y, Hada M (2008) An ab initio study based on a finite nucleus model for isotope fractionation in the U(III)–U(IV) exchange reaction system. J Chem Phys 128:144309
Abe M, Suzuki T, Fujii Y, Hada M, Hirao K (2010) Ligand effect on uranium isotope fractionations caused by nuclear volume effects: an ab initio relativistic molecular orbital study. J Chem Phys 133:044309
Almoukhalalati A, Shee A, Saue T (2016) Nuclear size effects in vibrational spectra. Phys Chem Chem Phys 18:15406–15417
Angeli I (2004) A consistent set of nuclear rms charge radii: properties of the radius surface R (N, Z). Atom Data Nucl Data Tables 87:185–206
Angeli I, Marinova KP (2013) Table of experimental nuclear ground state charge radii: an update. Atom Data Nucl Data Tables 99:69–95
Aufmuth P, Heilig K, Steudel A (1987) Changes in mean-square nuclear charge radii from optical isotope shifts. Atom Data Nucl Data Tables 37:455–490
Aurivillius K (1964) Least-squares refinement of the crystal structures of orthorhombic and HgO and of Hg2O2NaI. Acta Chem Scand 18:1305–1306
Baerends EJ et al (2019) Scientific computing and modelling (SCM), theoretical chemistry. Vrije Universiteit, Amsterdam
Bergquist BA, Blum JD (2007) Mass-dependent and -independent fractionation of Hg isotopes by photoreduction in aquatic systems. Science 318:417–419
Bigeleisen J (1996) Nuclear size and shape effects in chemical reaction. Isotope chemistry of the heavy elements. J Am Chem Soc 118:3676–3680
Bigeleisen J (1998) Second-order correction to the Bigeleisen-Mayer equation due to the nuclear field shift. Proc Natl Acad Sci USA 95:4908–4809
Bigeleisen J, Mayer MG (1947) Calculation of equilibrium constants for isotopic exchange reactions. J Chem Phys 15:261–267
Boher P, Garnier P, Gavarri JR, Hewat AW (1985) Monoxyde quadratique PbO alpha(I): Description de la transition structurale ferroelastique. J Solid State Chem 57:343–350
Bouvaist J, Weigel D (1970) Sesquioxyde de plomb, Pb2O3. I. Determination de la structure. Acta Crystallogr 26:501–510
Braekken H (1932) Die Kristallstruktur von Bleichlorid, PbCl2. Z Krist - Cryst Mater 83:222–226
Braekken H (1932) Zur Kristallstruktur des Quecksilberbromids HgBr2. Z Krist - Cryst Mater 81:152–154
Braekken H, Scholten W (1934) Die Kristallstruktur des Quecksilberchlorids HgCl2. Z Krist - Cryst Mater 89:448–455
Calos NJ, Kennard CHL, Davis RL (1989) The structure of calomel, Hg2Cl2, derived from neutron powder data. Z Krist - Cryst Mater 187:305–307
Chen JR, Nomura M, Fujii Y, Kawakami F, Okamoto M (1992) Gadolinium isotope separation by cation exchange chromatography. J Nucl Sci Technol 29:1086–1092
Dinnebier RE, Carlson S, Hanfland M, Jansen M (2003) Bulk modulus and high-pressure crystal structures of minium, Pb3O4. Am Mineral 88:996–1002
Downs RT, Hall-Wallace M (2003) The american mineralogist crystal structure database. Am Mineral 88:247–250
Ebert F, Woitinek H (1933) Kristallstrukturen von Fluoriden. II. HgF, HgF2, CuF und CuF2. Z Anorg Allg Chem 210:269–272
Estrade N, Carignan J, Sonke JE, Donard OFX (2009) Mercury isotope fractionation during liquid-vapor evaporation experiments. Geochim Cosmochi Acta 73:2693–2711
Fang T, Liu Y (2019) Equilibrium thallium isotope fractionation and its constraint on Earth’s late veneer. Acta Geochim 38(4):459–471
Franchini M, Philipsen PHT, Visscher L (2013) The Becke fuzzycCells integration scheme in the Amsterdam density functional program suite. J Comput Chem 34: 1818
Franchini M, Philipsen PHT, Lenthe E, Visscher L (2014) Accurate Coulomb potentials for periodic and molecular systems through densityfitting. J Chem Theory Comput 10:1994
Fricke G, Heilig K (2004) Group I: Element Particles, Nuclei and Atoms. 80-Hg Mercury. In: Boernstein L (ed) Numerical Data and Functional Relationships in Science and Technology new series. Springer, Heidelberg, pp 1–9
Fujii Y, Nomura M, Onitsuka H, Takeda K (1989) Anomalous isotope fractionation in uranium nrichment process. J Nucl Sci Technol 26:1061–1064
Fujii Y, Nomura M, Okamoto M, Onitsuka H, Kawakami F, Takeda K (1989) An anomalous isotope effect of 235U in U(IV)-U(VI) chemical exchange. Z Fur Naturforsch A 44:395–398
Fujii T, Yamamoto T, Inagawa J, Watanabe K, Nishizawa K (1998) Influences of nuclear size and shape and nuclear spin on chemical isotope effect of zirconium-crown complex. Ber Burtsenges Phys Chem 102:663–669
Fujii T, Inagawa J, Nishizawa K (1998) Influences of nuclear mass, size, shape and spin on chemical isotope effect of titanium. Ber Burtsenges Phys Chem 102:1880–1885
Fujii T, Kawashiro F, Yamamoto T, Nomura M, Nishizawa K (1999) Contribution of nuclear size and shape to chemical enrichment of iron isotopes. Solv Extr Ion Exch 17:177–190
Fujii T, Yamamoto T, Inagawa J, Gunji K, Watanabe K, Nishizawa K (1999) Nuclear size and shape effect in chemical isotope effect of gadolinium using dicyclohexano-18-crown-6. Solv Extr Ion Exch 17:1219–1229
Fujii T, Yamatomo T, Inagawaa J, Gunji K, Watanabe K, Nishizawa K (2000) Nuclear size and shape effects in chemical isotope enrichment of neodymium using a crown ether. Solv Extr Ion Exch 18:1155–1166
Fujii T, Suzuki D, Gunji K, Watanabe K, Moriyama H, Nishizawa K (2002) Nuclear field shift effect in the isotope exchange reaction of chromium(III) using a crown ether. J Phys Chem 106:6911–6914
Fujii T, Moynier F, Albarede F (2006) Nuclear field vs. nucleosynthetic effects as cause of isotopic anomalies in the early solar system. Earth Planet Sci Lett 247:1–9
Fujii T, Moynier F, Telouk P, Albarede F (2006) Mass-independent isotope fractionation of molybdenum and ruthenium and the origin of isotopic anomalies in Murchison. Astrophys J 647:1506–1516
Fujii T, Suzuki D, Yamana H (2008) Nuclear field shift effect of chromium(III) in repeated extraction using a crown ether. Solv Extr Ion Exch 26:100–112
Fujii T, Moynier F, Albarede F (2009) The nuclear field shift effect in chemical exchange reactions. Chem Geol 267:139–156
Fujii T, Moynier F, Telouk P, Albarede F (2009) Nuclear field shift effect in the isotope exchange reaction of cadmium using a crown ether. Chem Geol 267:157–163
Fujii T, Moynier F, Uehara A, Abe M, Yin QZ, Nagai T, Yamana H (2009) Mass-dependent and mass-independent isotope effects of zinc in a redox reaction. J Physl Chem A 113:12225–12232
Fujii T, Moynier F, Telouk P, Abe M (2010) Experimental and theoretical investigation of isotope fractionation of zinc between aqua, chloro, and macrocyclic complexes. J Phys Chem 114:2543–2552
Fujii T, Moynier F, Agranier A, Ponzevera E, Abe M (2011) Nuclear field shift effect of lead in ligand exchange reaction using a crown ether. Proc Radiochim Acta 1:387–392
Fujii T, Moynier F, Dauphas N, Abe M (2011) Theoretical and experimental investigation of nickel isotopic fractionation in species relevant to modern and ancient oceans. Geochim Cosmochim Acta 75:469–482
Fujii T, Moynier F, Agranier A, Ponzevera E, Abe M, Uehara A, Yamana H (2013) Nuclear field shift effect in isotope fractionation of thallium. J Radioanal Nucl Chem 296:261–265
Ghosh S, Xu XF, Humayun M, Odom L (2008) Mass-independent fractionation of mercury isotopes in the environment. Geochem Geophys Geosy 9:Q03004. https://doi.org/10.1029/2007GC001827
Ghosh S, Schauble EA, Couloume GL, Blum JD, Bergquist BA (2013) Estimation of nuclear volume dependent fractionation of mercury isotopes in equilibrium liquid-vapor evaporation experiments. Chem Geol 336:5–12
Grdenic D, Djordjevic C (1956) The Hg-Hg bond length in the mercurous ion. Part II. the crystal structure of mercurous fluoride. J Chem Soc 1956:1316–1319
Havighurst RJ (1925) Crystal structure of the mercurous halides. Am J Sci 10:15–28
Heilig K, Steudel A (1978). In: Hanel W, Kleinpoppen H (eds) Progress in atomic spectroscopy, part a. Plenum Press, New York, pp 263–328
Jain A, Ong SP, Hautier G, Chen W, Richards WD, Dacek S, Cholia S, Gunter D, Skinner D, Ceder G, Persson KA (2013) Commentary: The Materials Project: a materials genome approach to accelerating materials innovation. APL Mater 1:011002
James RW, Wood WA (1925) The crystal structures of barytes, celestine and anglesite. Proc R Soc Lond 109:598–620
King WH (1984) Isotope shifts in atomic spectra. Plenum Press, New York
Kolderup NH (1924) Crystal structure of fluorides of divalent metals. Mineral Abstr 3:340–340
Maley IJ, Parsons S, Pulham CR (2002) Lead(IV) chloride at 150 K. Acat Crystallograp 58:79–81
Méheut M, Ibañez-Mejia M, Tissot FLH (2020) Drivers of zirconium isotope fractionation in Zr-bearing phases and melts: the roles of vibrational, nuclear field shift and diffusive effects. Geochim Cosmochim Acta. https://doi.org/10.1016/j.gca.2020.09.028
Miller MF (2002) Isotopic fractionation and the quantifcation of O-17 anomalies in the oxygen three-isotope system: an appraisal and geochemical signifcance. Geochim Cosmochim Acta 66:1881–1889
Moynier F, Fujii T, Telouk P (2009) Mass-independent isotope fractionation of tin in chemical exchange reaction using a crown ether. Anal Chim Acta 632:234–239
Moynier F, Fujii T, Albarède F (2009) Nuclear field shift effect as a possible cause of Te isotopic anomalies in the early solar system—an alternative explanation of Fehr et al. (2006 and 2009). Meteorit Planet Sci 44(11):1735–1742
Moynier F, Fujii T, Brennecka GA, Nielsen SG (2013) Nuclear field shift in natural environments. C R Geoscience 345:150–159
Nemoto K, Abe M, Seino J, Hada M (2015) An ab intio study of nuclear volume effects for isotope fractionations using two-component relativistic methods. J Comput Chem 36:816–820
Nishizawa K, Nakamura K, Yamamoto T, Masuda T (1993) Zinc isotope effects in complex-formation with a crown-ether. Solv Extr Ion Exch 11:389–394
Nishizawa K, Nakamura K, Yamamoto T, Masuda T (1994) Separation of strontium and barium isotopes using a crown-ether. Different behaviors of odd mass and even mass isotopes. Solv Extr Ion Exch 12:1073–1084
Nishizawa K, Satoyama T, Miki T, Yamamoto T (1995) Strontium isotope effect in liquid-liquid extraction of strontium chloride using a crown ether. J Nucl Sci Technol 32:1230–1235
Nishizawa K, Maeda Y, Kawashiro F, Fujii T, Yamamoto T (1998) Contributions of nuclear size and shape, nuclear spin to enrichment factors of zinc isotopes in a chemical exchange reaction by a cryptand. Separ Sci Technol 33:2101–2112
Nomura M, Higuchi N, Fujii Y (1996) Mass dependence of uranium isotope effects in the U(IV)-U(VI) exchange reaction. J Am Chem Soc 118:9127–9130
Nowotny HN, Heger G (1986) Structure refinement of lead nitrate. Acta Crystallogr 42:133–135
Philipsen PHT, Velde G te, Baerends EJ, Berger JA, Boeij PL de, Franchini M, Groeneveld JA, Kadantsev ES, Klooster R, Kootstra F, Romaniello P, Raupach M, Skachkov DG, Snijders JG, Verzijl CJO, Gil JAC, Thijssen J M, Wiesenekker G, Ziegler T (2019) BAND2019, SCM, Theoretical Chemistry, Vrije Universiteit, Amsterdam, The Netherlands. http://www.scm.com
Quareni S, Pieri RD (1965) A three-dimensional refinement of the structure of crocoite, PbCrO4. Acta Crystallogr 19:287–289
Ramsdell LS (1925) The crystal structures of some metallic sulfides. Am Mineral 10:281–304
Schauble EA (2007) Role of nuclear volume in driving equilibrium stable isotope fractionation of mercury, thallium, and other very heavy elements. Geochim Cosmochim Acta 71:2170–2189
Schauble EA (2013) Modeling nuclear volume isotope effects in crystals. P Natl Acad Sci USA 110:17714–17719
Shibahara Y, Takaishi H, Nishizawa K, Fujii T (2002) Strontium isotope effects in ligand exchange reaction. J Nucl Sci Technol 39:451–456
Shibahara Y, Nishizawa K, Yasaka Y, Fujii T (2002) Strontium isotope effect in dmso-water system by liquid chromatography using a cryptand polymer. Solv Extr Ion Exch 20:67–79
Stacey DN (1966) Isotope shifts and nuclear charge distributions. Rep Prog Phys 29:171–215
Urey HC (1947) The thermodynamic properties of isotopic substances. J Chem Phys (London) 85:562–581
Velde G te, Baerends EJ (1991) Precise density-functional method for periodic structures. Phys Rev B 44:7888
Wiederhold JG, Cramer CJ, Daniel K, Infante I, Bourdon B, Kretzschmar R (2010) Equilibrium mercury isotope fractionation between dissolved Hg(II) species and thiol-bound Hg. Environ Sci Technol 44:4191–4197
Wiesenekker G, Baerends EJ (1991) Quadratic integration over the three-dimensional Brillouin zone. J Phys Condens Mat 3:6721
Yang S, Liu Y (2015) Nuclear volume effects in equilibrium stable isotope fractionations of mercury, thallium and lead. Sci Rep 5:12626. https://doi.org/10.1038/srep12626
Yang S, Liu Y (2016) Nuclear field shift effects on stable isotope fractionation: a review. Acta Geochim 35(3):227–239
Ye Y, Smyth JR, Boni P (2012) Crystal structure and thermal expansion of aragonite-group carbonates. Am Mineral 97:707–712
Young ED, Galy A, Nagahara H (2002) Kinetic and equilibrium mass-dependent isotope fractionation laws in nature and their geochemical and cosmochemical signifcance. Geochim Cosmochim Acta 66:1095–1104
Zachariasen WH (1926) Die kristallstruktur der telluride von zink, cadmium und quecksilber. Nor Geol Tidsskr 8:302–306
Zheng W, Hintelmann H (2009) Mercury isotope fractionation during photoreduction in natural water is controlled by its Hg/DOC ratio. Geochim Cosmochim Acta 73:6704–6715
Zheng W, Hintelmann H (2010) Nuclear field shift effect in isotope fractionation of mercury during abiotic reduction in the absence of light. J Phys Chem 114:4238–4245
Acknowledgements
This research was supported by National Natural Science Foundation of China (NSFC) projects (41703012) and Qinghai Science and Technology projects (2018-ZJ-956Q). Y.L is grateful for the supports of the Strategic Priority Research Program (B) of CAS (XDB18010100, XDB41000000), and pre-research Project on Civil Aerospace Technologies No. D020202 funded by the Chinese National Space Administration and NSFC projects (41530210).
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Yang, S., Liu, Y. Equilibrium mercury and lead isotope fractionation caused by nuclear volume effects in crystals. Acta Geochim 40, 150–162 (2021). https://doi.org/10.1007/s11631-021-00457-3
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11631-021-00457-3