Abstract
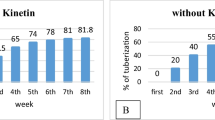
Dioscorea spp. is an important food crop in many countries and the source of the phytochemical diosgenin. Efficient microtuber production could provide source materials for farm-planting stock, for food markets, and for the production of high-diosgenin-producing cultivars. The first step in this study was optimizing the plant growth regulators for plantlet production, followed by a study of the effects of sucrose concentration on microtuber induction and diosgenin production. Significantly, more shoots (3.5) were produced at 4.65 μM (1 mg L−1) kinetin (KIN), longer shoots (4.1 cm) were obtained at 2.46 μM (0.5 mg L−1) indole-3-butyric acid (IBA), and root number (3.9) was significantly higher at 5.38 μM (1 mg L−1) naphthalene acetic acid (NAA) than in other treatments. Increased sucrose concentrations in the optimized growth medium with 4.65 μM KIN and 5.38 μM NAA had significant effects on microtuber production (p < 0.01) and diosgenin content (p < 0.05). The most microtubers (6.2) were obtained with 100 g L−1 sucrose, while those on 80 g L−1 sucrose were the heaviest (0.7 g) and longest (7.4 mm). Microtubers formed in medium with 80 g L−1 sucrose had significantly higher diosgenin content (3.64% [w/w]) than those in other sucrose treatments (< 2%) and was similar to that of field-grown parent tubers (3.79%). This result indicates an important role for sucrose in both microtuber growth and diosgenin production. Medium containing 4.65 μM KIN and 5.38 μM NAA is recommended for plantlet production, and medium containing 80 g L−1 sucrose is recommended for microtuber and diosgenin production.





Similar content being viewed by others
References
Adeniyi OJ, Adetimirin VO, Ingelbrecht I, Asiedu R (2008) Shoot and plantlet regeneration from meristems of Dioscorea rotundata Poir and Dioscorea alata L. Afr J Biotechnol 7:1003–1008
Aighewi BA, Asiedu R, Maroya N, Balogun M (2015) Improved propagation methods to raise the productivity of yam (Dioscorea rotundata Poir.). Food Security 7:823–834
Alexander J, Coursey DG (1969) The origins of yam cultivation. In: Ucko PJ, Dimbleby GW (eds) The domestication and exploitation of plants. Duckworth, London, pp. 405–425
Alizadeh S, Mantell SH, Viana AM (1998) In vitro shoot culture and microtuber induction in the steroidal yam Dioscorea composite Hemsl. Plant Cell Tissue Organ Cult 53:107–112
Balogun MO (2009) Microtubers in yam germplasm conservation and propagation: the status, the prospects and the constraints. Biotechnol Mol Biol Rev 4(1):1–10
Balogun MO, Fawole I, Ng SYC, Ng NQ, Shiwachi H, Kikuno H (2006) Interaction among cultural factors in microtuberization of white yam (Dioscorea rotundata). Trop Sci 46:55–59
Balogun MO, Maroya N, Asiedu R (2014) Status and prospects for improving yam seed systems using temporary immersion bioreactors. Afr J Biotechnol 13:1614–1622
Behera KK, Sahoo S, Prusti A (2009) Regeneration of plantlet of water yam (Dioscorea oppositifolia L.) through in vitro culture from nodal segments. Not Bot Hortic Agrobot Cluj 37(1):94–102
Chaturvedi HC, Jain H, Kidwai NR (2007) Cloning of medicinal plants through tissue culture—a review. Indian J Exp Biol 45:937–948
Das S, Choudhury MD, Mazumder PB (2013) In vitro propagation of genus Dioscorea—a critical review. Asian J Pharm Clin Res 6(Suppl 3):26–30
De Lourdes Contreras-Pacheco M, Santacruz-Ruvalcaba F, García-Fajardo JA, De Jesús Sánchez GJ, Ruíz LMA, Estarrón-Espinosa M, Castro-Castro A (2013) Diosgenin quantification, characterisation and chemical composition in a tuber collection of Dioscorea spp. in the state of Jalisco, Mexico. Int J Food Sci Technol 48:2111–2118
Dey P, Dutta S, Chaudhuri TK (2014) Phytochemical analysis of the leaves of Clerodendrum viscossum Vent. Int J Pharm Pharm Sci 6(2):254–258
Diarra ST, He J, Wang J, Li J (2013) Ethylene treatment improves diosgenin accumulation in in vitro cultures of Dioscorea zingiberensis via upregulation of CAS and HMGR gene expression. Electron J Biotechnol 16(5). doi:10.2225/vol16-issue5-fulltext-9
Donnelly DJ, Coleman WK, Coleman SE (2003) Potato microtuber production and performance: a review. Am J Potato Res 80:103–115
Ezeibekwe IO, Ezenwaka CL, Mbagwu FN, Unamba CIN (2009) Effect of combination of different levels of auxin (NAA) and cytokinin (BAP) on in vitro propagation of Dioscorea rotundata L. (white yam). J Mol Genet 1:18–22
FAO (2013) Statistical yearbook. Food and Agricultural Organization of the United Nations. Rome. www.fao.org/statistics/en/. Accessed 17 July 2014
Forsyth C, Van SJ (1982) An improved method of in vitro propagation of Dioscorea bulbifera. Plant Cell Tissue Organ Cult 1:275–281
Genstat (2010) Genstat for windows, 17th edn. VSN International Bioscience Software and Consultancy Ltd, Hemel Hempstead
Hostettmann KA, Marston A (1995) Saponins: chemistry and pharmacology of natural products. Cambridge University Press, Cambridge
Ishida BK (1988) Improved diosgenin production in Dioscorea deltoidea cell cultures by immobilization in polyurethane foam. Plant Cell Rep 7:270–273
Jamshidi S, Lahouti M, Ganjeali A (2014) Assessment of callus growth and bio-production of diosgenin in callus culture of Trigonella foenum-graecum L. Bull Env Pharmacol Life Sci 3(Special Issue 5):191–198
Kabeya MJ, Kabeya UC, Bekele BD, Kikuno H (2013) Vine cuttings technology in food yam (Discorea rotundata) production. Asian J Plant Sci Res 3(3):107–111
Kadota M, Niimi Y (2004) Improvement of micropropagation of Japanese yam using liquid and gelled medium culture. Sci Hortic 102:461–466
Klu GYP (2002) Conservation of Dioscorea rotundata: effect of basal medium type and naphthalene acetic acid on growth and microtuberization. West Afr J Appl Ecol 3:99–103
Lin JT, Yang DJ (2007) Determination of steroidal saponins in different organs of yam (Dioscorea pseudojaponica Yamamoto). Food Chem 108:1068–1074
Mahesh R, Muthuchelian K, Maridass M, Raju G (2010) In vitro propagation of wild yam, Dioscorea wightii through nodal cultures. Int J Biotechnol 1:111–113
Malaurie B, Thouvenel JC, Pungu O (1995) Influence of meristem-tip size and location on morphological development Dioscorea cayenensis–Dioscorea rotundata Complex ‘Grosse Caille’ and one genotype of Dioscorea praehensilis. Plant Cell Tissue Organ Cult 42:215–218
Mbanaso ENA, Chukwu LI, Opara MUA (2007) In vitro basal and nodal microtuberization in yam shoot cultures (Discorea rotundata Poir, cv. Obiaoturugo) under nutritional stress conditions. Afr J Biotechnol 6:2444–2446
Mert-Turk F (2006) Saponins versus plant fungal pathogens. J Cell Mol Biol 5:13–17
Murashige T, Skoog F (1962) A revised medium for rapid growth and bio assays with tobacco cultures. Physiol Plant 15:473–497
Nahapetian A, Bassiri A (1975) Changes in concentration and interrelationship of phytate, P, Mg, Cu, Zn, in wheat during maturation. J Agric Food Chem 32:1179–1182
Narula A, Kumar S, Srivastava PS (2005) Abiotic metal stress enhances diosgenin yield in Dioscorea bulbifera L. cultures. Plant Cell Rep 24:250–254
Okoli OO, Igbokwe MC, Ene LSC, Nwokoye JU (1982) Rapid multiplication of yam by the minisett technique. National Root Crop Research Institute, Umudike, Nigeria. Res Bull 2:1–12
Ovono PO, Kevers C, Dommes J (2010) Effects of storage conditions on sprouting of microtubers of yam (Dioscorea cayenensis–D. rotundata complex). C R Biol 333:28–34
Papadopoulou K, Melton RE, Leggett M, Daniels MJ, Osbourn AE (1999) Compromised disease resistance in saponin-deficient plants. Proc Natl Acad Sci U S A 96:12923–12928
Robertson GH, Doyle LR, Sheng P, Paviath AE, Goodman N (1989) Diosgenin formation by freely suspended and entrapped plant cell cultures of Dioscorea deltoidea. Biotechnol Bioeng 34:1114–1125
Rojas R, Alba J, Magana-Plaza I, Cruz F, Ramos-Valdivia AC (1999) Stimulated production of diosgenin in Dioscorea galeottiana cell suspension cultures by abiotic and biotic factors. Biotechnol Lett 21:907–911
Rokem JS, Tal B, Goldberg I (1985) Methods for increasing diosgenin production by Dioscorea cells in suspension cultures. J Nat Prod 48:210–222
Satour M, Mitaine-Offer AC, Lacaille-Dubois MA (2007) The Dioscorea genus: a review of bioactive steroid saponins. J Nat Med 61:91–101
Senanayake SA, Ranaweera KKDS, Bamunuarachchi A, Gunaratne A (2012) Proximate analysis and phytochemical and mineral constituents in four cultivars of yams and tuber crops in Sri Lanka. Trop Agric Res Ext 15(1):32–36
Suárez Padrón IE, Torres Arizal LA, Litz R (2011) Somatic embryogenesis in yam (Dioscorea rotundata). Revista Facultad Nacional de Agronomía Medellín 64:6037–6042
Supriya DAS, Manabendra DC, Pranab BM (2013) Micropropagation of Dioscorea alata through nodal segments. Afr J Biotechnol 12:6611–6617
Yeh FT, Huang WW, Cheng CC, Na C, Tsay HS (1994) Tissue culture of Dioscorea doryophora Hance. II. Establishment of suspension culture and the measurement of diosgenin content. Chinese Agron J 4:257–268
Yi T, Fan LL, Chen HL, Zhu GY, Suen HM, Tang YN, Zhu L, Chu C, Zhao ZZ, Chen HB (2014) Comparative analysis of diosgenin in Dioscorea species and related medicinal plants by UPLC-DAD-MS. BMC Biochem 15:19. doi:10.1186/1471-2091-15-19
Zhu YL, Huang W, Ni J, Liu W, Li H (2010) Production of diosgenin from Dioscorea zingiberensis tubers through enzymatic saccharification and microbial transformation. Appl Microbiol Biotechnol 85:1409–1416
Acknowledgements
This study was carried out with the facility and materials provided by the Department of Agronomy, University of Ibadan, Nigeria. Funds for the upgrade of the laboratory were provided by the Alliance for a Green Revolution in Africa (AGRA), Nairobi, Kenya; the National Biotechnology Development Agency (NABDA), Abuja, Nigeria; and the University of Ibadan, Ibadan, Nigeria. The authors would also like to thank IITA for providing the plant materials used for these studies.
Author information
Authors and Affiliations
Corresponding author
Additional information
Editor: Todd Jones
Rights and permissions
About this article
Cite this article
Uchendu, E.E., Sobowale, O.O., Odimegwu, J. et al. In vitro sucrose concentration influences microtuber production and diosgenin content in white yam (Dioscorea rotundata Poir). In Vitro Cell.Dev.Biol.-Plant 52, 563–570 (2016). https://doi.org/10.1007/s11627-016-9789-y
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11627-016-9789-y