Abstract
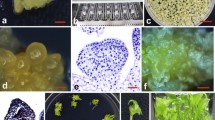
In banana and plantain research, it is essential to establish embryogenic cell suspensions together with a highly efficient regeneration and transformation system. This article describes the development of an embryogenic cell suspension (ECS), regeneration, and transformation for plantain cv. “Gonja manjaya”. ECS was established using highly proliferative multiple buds. The frequency of embryogenic friable callus formation was 56.8% of the cultured explants. Friable embryogenic calli with many translucent proembryos were transferred to liquid medium and homogenous cell suspensions were established within 3–4 mo. Approximately 25,000 to 30,000 plants per 1.0 ml of settled cell volume were regenerated in approximately 13–14 mo. ECSs were transformed using Agrobacterium strain EHA 105 harboring the binary vector pBI121. About 50–60 transgenic plants per 0.5 ml settled cell volume were regenerated on selective medium containing 100 mg l−1 kanamycin. Histochemical GUS assays using different tissues of putatively transformed plants demonstrated stable expression of uidA gene. The presence and integration of the uidA gene were confirmed by PCR and Southern blot analysis, respectively. This is the first report showing establishment of embryogenic cell suspension cultures and Agrobacterium-mediated transformation of an important plantain cultivar, “Gonja manjaya”. This study shows the huge potential for genetic transformation of plantains for disease or pest resistance, as well as tolerance to abiotic factors such as drought stress using this robust regeneration and transformation protocol.




Similar content being viewed by others
References
Arinaitwe G.; Remy S.; Strosse H.; Swennen R.; Sági L. Agrobacterium- and particle bombardment-mediated transformation of a wide range of banana cultivars. In: Jain S. M.; Swennen R. (eds) Banana Improvement, cellular, molecular biology and induced mutations. Science Publishers Inc., Enfield, pp 351–358; 2004.
Becker D. K.; Dugdale B.; Smith M. K.; Harding R. M.; Dale J. L. Genetic transformation of Cavendish banana (Musa spp. AAA group) cv. ‘Grand Nain’ via microprojectile bombardment. Plant Cell Rep 19: 229–234; 2000.
Bretagne-Sagnard B.; Chupeau Y. Selection of transgenic flax plants facilitated by spectinomycin. Transgene Res 5: 131–137; 1996.
Côte F. X.; Domergue R.; Monmarson S.; Schwendiman J.; Teisson C.; Escalant J.-V. Embryogenic cell suspensions from the male flower of Musa AAA cv. Grand Nain. Physiol Plant 97: 285–290; 1996.
Dheda D.; Dumortier F.; Panis B.; Vuylsteke D.; Langhe E. Plant regeneration in cell suspension cultures of the cooking banana cv. Bluggoe (Musa spp. ABB group). Fruits 46: 125–135; 1991.
Escalant J.-V.; Teisson C.; Cote F. Amplified somatic embryogenesis from male flowers of triploid banana and plantain cultivars (Musa spp.). In Vitro Cell Dev Biol 30: 181–186; 1994.
FAOSTAT (Food and Agriculture Organization of the United Nations). FAOSTAT Agriculture Data http://faostat.fao.org; Cited 2004.
FAOSTAT. FAOSTAT Agriculture Data. http://faostat.fao.org; Cited 2009.
Ganapathi T. R.; Higgs N. S.; Balint-Kurti P. J.; Arntzen C. J.; May G. D.; Van Eck J. M. Agrobacterium-mediated transformation of the embryogenic cell suspensions of the banana cultivars Rasthali (AAB). Plant Cell Rep 20: 157–162; 2001.
Gawel N. J.; Jarret R. L. A modified CTAB DNA extraction procedure for Musa and Ipomoea. Plant Mol Biol Rep 9: 262–266; 1991.
Gelvin S. B. Agrobacterium-Mediated Plant Transformation: the Biology behind the “Gene-Jockeying” Tool. Microbiol Mol Biol Rev 67: 16–37; 2003.
Gheysen G.; Angenon G.; Montague M. V. Agrobacterium-mediated plant transformation: a scientifically intriguing story with significant application. In: Lindsey K. (ed) Transgenic plant research. Harwood Academic, Amsterdam, pp 1–33; 1998.
Ghosh A.; Ganapathi T. R.; Nath P.; Bapat V. A. Establishment of embryogenic cell suspension cultures and Agrobacterium-mediated transformation in an important Cavendish banana cv. Robusta (AAA). Plant Cell Tissue Organ Cult 97: 131–139; 2009.
Grapin A.; Ortíz J.-L.; Lescot T.; Ferrière N.; Côte F. X. Recovery and regeneration of embryogenic cultures from female flowers of False Horn Plantain. Plant Cell Tissue Organ Cult 61: 237–244; 2000.
Hansen G.; Wright M. S. Recent advances in transformation of plants. Trends Plant Sci 4: 226–231; 1999.
Hood E. E.; Gelvin S. B.; Melchers S.; Hoekema A. New Agrobacterium plasmids for gene transfer to plants (EHA 105). Trans Res 2: 208–218; 1993.
Jalil M.; Khalid N.; Othman R. Y. Plant regeneration from embryogenic suspension cultures of Musa acuminata cv. Mas (AA). Plant Cell Tissue Organ Cult 75: 209–214; 2003.
Jefferson R. A. Assaying chimeric genes in plants: The GUS gene fusion system. Plant Mol Biol Rep 5: 387–405; 1987.
Khanna H.; Becker D.; Kleidon J.; Dale J. Centrifugation Assisted Agrobacterium tumefaciens-mediated Transformation (CAAT) of embryogenic cell suspensions of banana (Musa spp. Cavendish AAA and Lady Finger AAB). Mol Breed 14: 239–252; 2004.
Kosky R. G.; Chong-Pérez B.; López-Torres J.; Reyes M.; Bermúdez-Caraballoso I.; Martín N. M.; Machado-Rodriguez J. M.; Portal O.; Ocaña B.; Alvarado-Capó Y.; Leiva-Mora M.; Acosta-Suárez M.; Cruz-Martin M.; Roque B.; Hernández L. Plantain (Musa spp. cv. ‘Navolean’ AAB) transgenic plants from Agrobacterium tumefaciens-mediated transformation of embryogenic cell suspensions. Biotecnología Vegetal 10: 209–218; 2010.
Lescot T. Genetic diversity of banana in figures. FruiTrop 155: 29–33; 2008.
Lindsey K. Genetic manipulation of crop plants. J Biotechnol 26: 1–28; 1992.
Lorenzen J.; Tenkouano A.; Bandyopadhyay R.; Vroh B.; Coyne D.; Tripathi L. Overview of Banana and Plantain (Musa spp.) Improvement in Africa: Past and Future. Acta Hortic 879: 595–603; 2010.
Marroquin C. G.; Paduscheck C.; Escalant J. V.; Teisson C. Somatic embryogenesis and plant regeneration through cell suspensions in Musa acuminate. In Vitro Cell Dev Biol 29: 43–46; 1993.
May G. D.; Rownak A.; Mason H.; Wiecko A.; Novak F. J.; Arntzen C. J. Generation of transgenic banana (Musa acuminata) plants via Agrobacterium-mediated transformation. Bio/Technol 13: 486–492; 1995.
Morais-Lino L. S.; Santos-Serejo J. A.; Oliveira e Silva S.; Ferreira de Santana J. R.; Kobayashi A. K. Cell suspension culture and plant regeneration of a Brazilian plantain, cultivar Terra. Pesqui Agropecu Bras 43: 1325–1330; 2008.
Morel G.; Wetmore R. H. Tissue culture of monocotyledons. Amer J Bot 38: 138–140; 1951.
Murashige T.; Skoog F. A revised medium for rapid growth and bioassays with tobacco tissue cultures. Physiol Plant 15: 473–497; 1962.
Navarro C.; Escobedo R. M.; Mayo A. In vitro plant regeneration from embryogenic cultures of a diploid and a triploid. Cavendish banana. Plant Cell Tissue Organ Cult 51: 17–25; 1997.
Novak F. J.; Afza R.; Van Duren M.; Perea-Dallos M.; Conger B. V.; Xiaolang T. Somatic embryogenesis and plant regeneration in suspension cultures of dessert (AA and AAA) and cooking (ABB) bananas (Musa spp.). Bio/Technol 7: 147–158; 1989.
Panis B.; Withers L. A.; De Langhe E. Cryopreservation of Musa suspension cultures and subsequent regeneration of plants. Cryo-Letters 11: 337–350; 1990.
Sagi L.; Panis B.; Remy S.; Schoofs H.; De Smet K.; Swennen R.; Cammus B. Genetic transformation of banana (Musa spp.) via particle bombardment. Bio/Technol 13: 481–485; 1995.
Schenk R. U.; Hildebrandt A. C. Medium and techniques for induction and growth of monocotyledonous and dicotyledonous plant cell cultures. Can J Bot 50: 199–204; 1972.
Shibata D.; Liu Y.-G. Agrobacterium-mediated plant transformation with large DNA fragments. Trends Plant Sci 5: 354–357; 2000.
Strosse H.; Schoofs H.; Panis B.; Andre E.; Reyniers K.; Swennen R. Development of embryogenic cell suspensions from shoot meristematic tissue bananas and plantains (Musa spp.). Plant Sci 170: 104–112; 2006.
Tripathi L.; Mwaka H.; Tripathi J. N.; Tushemereirwe W. K. Expression of sweet pepper Hrap gene in banana enhances resistance to Xanthomonas campestris pv. musacearum. Mol Plant Pathol 11: 721–731; 2010.
Tripathi L.; Tripathi J. N.; Hughes J. d’ A. Agrobacterium-mediated transformation of plantain (Musa spp.) cultivar Agbagba. African J Biotechnol 4: 1378–1383; 2005.
Tripathi L.; Tripathi J. N.; Oso R. T.; Hughes J. d’ A; Keese P. Regeneration and transient gene expression of African Musa species with diverse genomic constitution and ploidy levels. Tropical Agr 80: 182–187; 2003.
Tripathi L.; Tripathi J. N.; Tushemereirwe W. K. Rapid and efficient production of transgenic East African Highland Banana (Musa spp.) using intercalary meristematic tissues. African J Biotechnol 7: 1438–1445; 2008.
Viljoen A. Protecting the African Banana (Musa spp.): Prospects and Challenges. Acta Hortic 879: 305–314; 2010.
Vuylsteke D. Breeding bananas and plantains: from intractability to feasibility. Acta Hortic 540: 453–459; 2000.
Yao J.-L.; Cohen D.; Atkinson R.; Richardson K.; Morris B. Regeneration of transgenic plants from the commercial apple cultivar Royal Gala. Plant Cell Rep 14: 407–412; 1995.
Acknowledgments
The authors would like to thank the African Agriculture Technology Foundation (AATF) and Gatsby Charitable Foundation for funding support for this work and the National Agriculture Research Laboratories, Uganda for providing the laboratory facilities.
Author information
Authors and Affiliations
Corresponding author
Additional information
Editor: John W. Forster
Rights and permissions
About this article
Cite this article
Tripathi, J.N., Muwonge, A. & Tripathi, L. Efficient regeneration and transformation of plantain cv. “Gonja manjaya” (Musa spp. AAB) using embryogenic cell suspensions. In Vitro Cell.Dev.Biol.-Plant 48, 216–224 (2012). https://doi.org/10.1007/s11627-011-9422-z
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11627-011-9422-z