Abstract
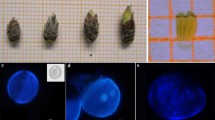
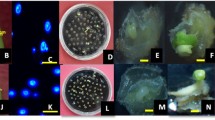
Plant regeneration was obtained from cultured anthers and hypocotyl segments of caraway (Carum carvi L.). Microspore- and somatic tissue-derived embryos were compared by observation of the regeneration process under identical induction conditions. Fluorescent microscopy with DAPI staining showed initiation of cell divisions and formation of embryogenic callus and somatic embryos from anther sacs, with production of embryos of both microspore and somatic origin. Induction of somatic embryos from hypocotyl-derived callus was also demonstrated. Isozyme native polyacrylamide gel electrophoresis was used to identify haploids and doubled haploids, and to determine the frequency of spontaneous diploidization of regenerated plants of microspore origin. Donor plants (2n = 20) and their anther-derived derivative plants (n = 10, 2n = 20, 4n = 40) in callus stage or leafy rosette stage were compared. The esterase (EST) band patterns of regenerated plants differed from the heterozygous parental material, suggesting that the regenerated plants were microspore-derived haploid/doubled haploid plants. The similar profile of EST bands between the diploid anther-derived plants and a sample of the donor plants corresponded to a somatic regeneration pathway. Although the selected induction conditions revealed no preference for induction of microspore embryogenesis, the anther culture protocol established for caraway utilizing isozyme segregating EST loci markers is suitable for DH production.




Similar content being viewed by others
References
Acquaah G. Practical protein electrophoresis for genetic research. Chapter I. Genetic Principles Associated Isozymes. Dioscorides Press, Portland, pp 13–15; 1992.
Adamus A.; Michalik B. Anther cultures of carrot (Daucus carota L.). Folia. Hort. Ann. 15: 49–58; 2003.
Ammirato P. V. Embryogenesis. In: Evans D. A.; Sharp W. R.; Ammirato P. V.; Yamada Y. (eds) Handbook of Plant Cell Culture, Techniques for Propagation and Breeding, vol 1. MacMillan Publishing Company, New York, pp 82–123; 1993.
Andersen S. B.; Christiansen I.; Farestveit B. Carrot (Daucus carota L.): In vitro production of haploids and field trails. In: Bajaj Y. P. S. (ed) Biotechnology in agriculture and forestry, Haploids in crop improvement I, vol 12. Springer, Berlin, Germany; Heidelberg, Germany; New York, NY, USA, pp 393–402; 1990.
Aulinger I. E.; Peter S. O.; Schmid J. E.; Stamp P. Rapid attainment of a doubled haploid line from trasgenic maize (Zea mays L.) plants by means of anther culture. In Vitro Cell Dev. Biol. Plant 39: 165–170; 2003.
Baranski R. Application of a rapid glucose-6-phosphate isomerase assay in white cabbage breeding. Acta. Physiol. Plant 22: 45–51; 2000.
Bartošová Z.; Oberť B.; Takáč T.; Kormuťák A.; Preťová A. Using enzyme polymorphism to identify the gametic origin of flax regenerants. Acta. Biolog. Crac. Ser. Botan 47: 173–178; 2005.
Datta S. K. Androgenic haploids: Factors controlling development and its application in crop improvement. Curr. Sci. 89: 1870–1878; 2005.
Ferrie A. M. R. Current status of doubled haploids in medicinal plants. In: Touraev A.; Forster B. P.; Jain S. M. (eds) Current status of doubled haploids in medicinal plants advances in haploid production in higher plants. Springer, Berlin, Germany; Heidelberg, Germany; New York, NT, USA, pp 209–217; 2009.
Ferrie A. M. R.; Bethune T.; Kernan Z. An overview of preliminary studies on the development of doubled haploid protocols for nutraceutical species. Acta. Physiol. Plant 27: 735–741; 2005.
Ferrie A. M. R.; Mykytyshyn M. L.; Bethune T. Methods for producing microspore derived doubled haploid apiaceae. US patent no. US 2009/0100538 A1; 2009.
Furmanova M.; Oledzka H.; Sowinska D. Regeneration of plants by embryogenesis with callus cultures of Carum carvi L. J. Plant. Physiol. 115: 209–210; 1984.
Gamborg O. L.; Miller R. A.; Ojima K. Nutrient requirements of suspension cultures of soybean root cells. Exp Cell Res 50: 151–158; 1968.
Gorećka K.; Kowalska U.; Krzyzanovska D.; Gorećki R. Obtaining carrot (Daucus carota L.) plants in isolated microspore cultures. J. Appl. Genet. 51: 141–147; 2010.
Gorećka K.; Krzyzanovska D.; Gorećki R. The influence of several factors on the efficiency of androgenesis in carrot. J. Appl. Genet. 46: 265–269; 2005.
Gottlieb L. D. Electrophoretic evidence and plant populations. Prog. Phytochem. 7: 1–46; 1981.
Heberle-Bors E.; Reinert J. Androgenesis in isolated pollen cultures of Nicotiana tabacum: dependence upon pollen development. Protoplasma 99: 237–245; 1979.
Hussein M. A.; Batra A. In vitro embryogenesis of cumin hypocotyl segments. Ad. Plant. Sci 11: 125–127; 1998.
Jimenez V. M. Involvement of plant hormones and plant growth regulators on in vitro somatic embryogenesis. Plant. Growth. Regul 47: 91–110; 2005.
Kim M.; Kim J.; Yoon M.; Choi D. I.; Lee K. M. Origin of multicellular pollen and pollen embryos in cultured anthers of pepper (Capsicum annuum). Plant. Cell. Tiss. Org. Cult 77: 63–72; 2004.
Krens F. A.; Keizer L. C. P.; Capel I. E. M. Transgenic caraway, Carum carvi L.: a model species for metabolic engineering. Plant. Cell. Rep 17: 39–43; 1997.
Matsubara S.; Dohya N.; Murakami K. Callus formation and regeneration of adventitious embryos from carrot, fennel and mitsuba microspores by anther and isolated microspore cultures. Act. Horticult 392: 129–137; 2005.
Nemeth E. Caraway. The Genus Carum. Harwood Academic Publishing, Amsterdam; 1998.
Palmer C. E.; Keller W. A.; Kasha K. J. Biotechnology in agriculture and forestry. Haploids in crop improvement. vol 56. Springer, Berlin, Germany; Heidelberg, Germany; New York, NY, USA; 2005.
Rodriques L. R.; Oliviera J. M. S.; Mariath J. E. A.; Bodanese-Zanettini M. H. Histology of embryogenic responses in soybean anther culture. Plant. Cell. Tiss. Org. Cult 80: 129–137; 2005.
Schmidt E. D. L.; Guzzo F.; Toonen M. A. J.; De Vries S. C. A leucine-rich repeat containing receptor-like kinase marks somatic plant cells competent to form embryos. Development 124: 2049–2062; 1997.
Shukla M. R.; Subhash N.; Patel D. R.; Patel S. A. In vitro studies in cumin (Cuminum cyminum L.). In: Edison S.; Ramana K. V.; Sasikumar B.; Nirmal B. K.; Eapen S. J. (eds) Biotechnology of Spices, Medicinal and Aromatic Plants. Indian Society for Spices, Calicut, pp 45–48; 1997.
Smýkal P. Pollen embryogenesis—the stress mediated switch from gametophytic to sporophytic development. Current status and future prospects. Biol. Plant 43: 481–489; 2000.
Smýkalová I.; Šmirous P.; Kubošiová M.; Gasmannová N.; Griga M. Doubled haploid production via anther culture in annual, winter type of caraway (Carum carvi L.). Acta. Physiol. Plant 31: 21–31; 2009.
Sopory S. K.; Munshi M. Anther culture. In: Jain S. M.; Sopory S. K.; Veilleux R. E. (eds) In vitro haploid Production in Higher Plants, Applications, vol. 2. Kluwer Academic Publishers, Dordrecht, The Netherlands, pp 145–176; 1996.
Tawfik A. A.; Noga G. Adventitious shoot proliferation from hypocotyl and internodal stem explants of cumin. Plant. Cell. Tiss. Org. Cult 66: 141–147; 2001.
Touraev A.; Forster B. P.; Jain S. M. Advances in haploid production in higher plants. Springer, Berlin, Germany; Heidelberg, Germany; New York NY, USA; 2009.
Vallejos C. E. Enzyme activity staining. In: Tanksley S. D., Orton T. J. (eds) Isozymes in plant genetic and breeding, part A. Elsevier, Amsterdam, The Netherlands; Oxford, England; New York, NT, USA: 469–516; 1983.
Wang M.; Van Bergen S.; Van Duijn B. Insights into a key developmental switch and its importance for efficient plants breeding. Update on plant development. Plant Physiol 124: 523–530; 2000.
Weeden N. F.; Gottlieb L. D. Distinguishing allozymes and isozymes of phosphoglucoisomerases by electrophoretic comparisons of pollen and somatic tissues. Biochem. Genet. 17: 287–296; 1979.
Zhao J. P.; Simmonds D. H.; Newcomb W. Induction of embryogenesis with colchicine instead of heat in microspores of Brassica napus L. Cv. Topas. Planta 198: 433–439; 1996.
Acknowledgments
This study was supported by grants from the Ministry of Agriculture of the Czech Republic (no. QF 4056) and the Ministry of Education, Youth and Sports of the Czech Republic (no. MSM 6198959216 and MSM 6198959215). The authors are grateful to Drs. Allison Ferrie and Darren Eve for the critical reading of the manuscript and for valuable comments.
Author information
Authors and Affiliations
Corresponding author
Additional information
Editor: J. Finer
Electronic supplementary materials
Below is the link to the electronic supplementary material.
ESM 1
(PPT 44338 kb)
Rights and permissions
About this article
Cite this article
Smýkalová, I., Horáček, J., Kubošiová, M. et al. Induction conditions for somatic and microspore-derived structures and detection of haploid status by isozyme analysis in anther culture of caraway (Carum carvi L.). In Vitro Cell.Dev.Biol.-Plant 48, 30–39 (2012). https://doi.org/10.1007/s11627-011-9386-z
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11627-011-9386-z