Summary
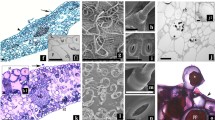
The anatomy of normal and hyperhydric in vitro shoots and leaves from micropropagated simmondsia chinensis (Link.) Schn. (jojoba) was compared with that of seedlings (control plants). In vitro normal plantlets displayed good development and survived during the acclimatization stage. In vitro hyperhydric plantlets presented numerous anatomical defects, such as hypertrophy of the mesophyll and of the stem cortex, malformed non-functional stomata, epidermal discontinuity, and xylem hypolignification; they did not survice acclimatization. The study of the anatomical features of in vitro jojoba shoots and leaves allowed determination of the structural condition of the plantlets and prediction of which plantlet would survive the critical acclimatization stage.
Similar content being viewed by others
References
Apóstolo, N. M.; Llorente, B. E. Enraizamiento y rusticación de plántulas de jojoba micropropagadas. In: Prince, L. H.; Rossi, C., eds. Proceedings of IX International Conference on jojoba and its uses, Catamarea, Argentina, Association for Advancement of Industrial Crops. East Peoria, USA: Versa Press; 1996; 47–49.
Aragão, R. G. M.; Hogan, L. M. Crescimiento e differenciaçã de tecidoes de jojoba. Ciéncia Agronomica 6:75–84; 1976.
Ayerza, Rh. El cultivo de jojoba. Revista Oleaginosas (April):38–41; 1992.
Baas, P.; Gregory, M. A survey of oil cells in the Dicotyledons with comments on their replacement by and joint occurrence with mucilage cells. Israel J. Bot. 34:167–186; 1985.
Birnbaum, E.; Matias, S.; Wenkert, C. Vegetative propagation of jojoba by tissue culture. In: Wisniak, J.; Zabicky, J., eds Proceedings of VI International Conference on jojoba and its uses, Ben Gurion University, Beer-Sheva, Israel. 1984; 233–241.
Chaturvedi, H.; Sharma, M. In vitro production of cloned plants of jojoba through shoot proliferation in long term culture. Plant Sci. 63: 199–207; 1989.
Cohen, A. L.: Critical point drying. Principles and procedures. Scand. Electr. Microsc. 11:303–324; 1979.
D'Ambrogio, A. Manual de ténicas en hitología vegetal Argentina: Hemisferio Sur; 1986.
Debergh, P.; Aitken-Christie, J.; Cohen, D.; Grout, B.; von Arnold, S.; Zimmerman, R.; Ziv, M. Reconsideration of the term vitrification as used in micropropagation. Plant Cell Tiss. Org. Cult. 30: 135–140; 1992.
Gregory, M.; Baas, P. A survey of mucilage cells in vegetative organs of the Dicotyledons. Israel J. Bot. 38: 125–174; 1989.
Gribble, K.; Sarafis, V; Nailon, J.; Holford P.; Uwins, P. Environmental scanning electron microscopy of the surface of normal and vitrified leaves of Gypsophila paniculata (babies' breath) cultures in vitro. Plant Cell Rep. 15:771–776; 1996.
Johansson, M.; Kronestedt-Robards, E. C.; Robards, A. W. Rose leaf structure in relation to different stages of micropropagation. Protoplasma 166:165–176; 1992.
Lee, C. W. Application of biotechnology for clonal propagation and yield enhancement in jojoba. In: Baldwin, A. R., ed. Proceedings of VII International Conference on jojoba and its uses. Illinois: Academican Oil Chemists Society; 1988; 102–110.
Llorente, B. E.; Apóstolo, N. M. Micropropagación dejojoba: efecto de la variación hormonal y clonal en la etapa de multiplicación. In: Prince L. H.; Rossi, C., eds. Proceedings of IX International Conference on jojoba and its uses, Catamarca Argentina, Association for Advancement of Industrial Crops. East Peoria USA: Versa Press; 1996; 50–52.
Llorente, B. E.; Apóstolo, N. M. Effect of different growth regulators and genotype on in vitro propagation of jojoba. New Zealand J Hort. Sci. 26:55–62; 1998.
Mercure, E. W.; Jones, C. S.; Brand, M. H.; Auer, C. A. Anatomy of shoots and tumors of in vitro habituated Rhododendron ‘Montego’ (Ericaceae) cultures with tissue proliferation. Amer. J. Bot. 85:616–628; 1998.
Noé, N.; Bonini, L. Leaf anatomy of highbush blueberry grown in vitro and during acclimatization to ex vitro conditions. Biol. Plantarum 38:19–25; 1996.
Olmos, E.; Hellín, E. Ultrastructural differences of hyperhydric and normal leaves from regenerated carnation plants. Scientia Hort. 75:91–101; 1998.
Sallanon, H.; Tort, M.; Coudret, A. The ultrastructure of micropropagated and greenhouse rose plant stomata. Plant Cell Tiss. Org. Cult. 32:227–233; 1993.
Scaramuzzi, F.; D'Ambrosio, A. Organogenesis and propagation in vitro of Simmondsia chinensis (Link) Schn. jojoba from vegetative fragments. Acta Hort. 227:411–413; 1988.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Apóstolo, N.M., Llorente, B.E. Anatomy of normal and hyperhydric leaves and shoots of in vitro grown Simmondsia chinesis (link) schn. In Vitro Cell.Dev.Biol.-Plant 36, 243–249 (2000). https://doi.org/10.1007/s11627-000-0045-z
Received:
Accepted:
Issue Date:
DOI: https://doi.org/10.1007/s11627-000-0045-z