Abstract
Microphysiological systems (MPS) designed to study the complexities of the peripheral and central nervous systems have made marked improvements over the years and have allowed researchers to assess in two and three dimensions the functional interconnectivity of neuronal tissues. The recent generation of brain organoids has further propelled the field into the nascent recapitulation of structural, functional, and effective connectivities which are found within the native human nervous system. Herein, we will review advances in culture methodologies, focused especially on those of human tissues, which seek to bridge the gap from 2D cultures to hierarchical and defined 3D MPS with the end goal of developing a robust nervous system-on-a-chip platform. These advances have far-reaching implications within basic science, pharmaceutical development, and translational medicine disciplines.
Similar content being viewed by others
Introduction
Microphysiological systems (MPS) designed to study the complexities of the peripheral and central nervous system have made marked improvements over the years and have allowed researchers to assess in two and three dimensions the functional interconnectivity of neuronal tissues. The recent generation of brain organoids has further propelled the field into the nascent recapitulation of structural, functional and effective connectivity which is found within the native human nervous system. Herein, we will review advances in culture methodologies, focused especially on those of human tissues, which seek to bridge the gap from 2D cultures to hierarchical and defined 3D MPS with the end goal of developing a robust nervous system-on-a-chip platform. Rather than provide a comprehensive review on this extensive subject, we have endeavored to highlight a number of important design considerations that are especially important for developing models of the nervous system. We discuss features including substrate and tissue mechanics, inclusion of neuron and glial subtypes, cell-cell interactions, representative histoarchitecture, functional assessments, and the emerging importance of 3D configuration. We then turn to how those design features are being implemented specifically in models of the central nervous system and of peripheral nerve. We then briefly discuss some translational applications of these models and conclude with a discussion of improvements that are expected in key areas with a nod toward future development. These advances have far-reaching implications within basic science, pharmaceutical development, and translational medicine disciplines.
Current Challenges in Drug Development
Millions of Americans suffer from neurological disorders such as Alzheimer’s disease, Parkinson’s disease, multiple sclerosis (MS) and amyotrophic lateral sclerosis (ALS) and novel therapeutics are necessary to treat said conditions. Globally, in 2016, neurological disorders were the leading cause of disability-adjusted life years (276 million) and second leading cause of deaths (9 million) (Feigin and Vos 2019). Approximately 15% of children in the US ages 3 to 17 yr were affected by neurodevelopmental disorders (Environmental Protection Agency 2015). Notably, the opioid crisis within the United States of America has resulted in astronomical social and financial burdens with a running total of over 750,000 deaths (Centers for Disease Control 2020) and more than $72.4 billion spent related to opioid use disorder over 15 yr (Leslie et al. 2019) spent on care. To both overcome this current crisis and address the broader need for streamlining within the drug development pipeline, more physiologically relevant models are needed to screen potential candidate compounds in nascent testing stages. The overall goal should be to rule out cytotoxic drugs and drugs without human efficacy earlier, thus reducing time and money spent on determining appropriate candidates.
While poor launch probability is pervasive across all medical disciplines, drugs developed targeting nervous system disorders are the least likely to succeed beyond phase I and phase II clinical trials and only cardiovascular disease is ahead for phase III attrition (Dowden and Munro 2019). The failure rate is especially high for neurodegenerative drugs where, e.g., 99.6% of experimental drugs for Alzheimer’s disease have not made it to the market (Pistollato et al. 2016; Mohs and Greig 2017). Further, most treatments for central nervous system disorders do not affect the course of the disease but rather serve as palliative therapy. Deeper understandings in neuronal and glial functionality that can be studied using discrete, testable three-dimensional microphysiological systems are imperative to the development of therapeutics that go beyond this limitation of symptom management and move more towards regenerative therapies.
Neural MPS Tissue Design Considerations
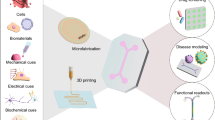
The advent of stem cell technologies and bioengineering in cell culture have led to the development of organotypic cultures, i.e., organoids, organ-on-chip, and human-on-a-chip (multiorgan) approaches, jointly termed microphysiological systems as they replicate some organ architecture and physiology (Figs. 1 and 2). This term (MPS) encompasses the use of various culture and engineering strategies, such as the aforementioned methods, and differs from basic cell culture techniques by seeking to incorporate aspects of the tissue microenvironment to recapitulate some organ-level functionality without requiring in situ or in vivo methods. Their status was summarized in two stakeholder workshops (Marx et al. 2016, 2020). Implementing these organotypic cultures within robust microphysiological systems requires the consideration of a multitude of factors that contribute to the success of the system as determined by the physiological relevance and ultimate translatability. Key enabling features to consider include overall structure, individual and population cell:cell interactions, and functional readouts necessary to answer discrete, testable hypotheses. These biomechanical, biochemical, architectural and cell-based cues all contribute to the recapitulation of niches found within the nervous system with each factor working in concert with another. Here we outline examples of advancements made from tuning these key enabling features of MPS designs towards nervous system modeling.
Three-dimensional brain organoid models. Advances in culture methodologies have allowed researchers to build complex, heterogenous brain organoids that are both structurally similar to in vivo architectures and provide meaningful functional readouts. Brain organoids, over traditional two-dimensional cultures, can integrate a myriad of neuronal and glial cell types which exhibit histoarchitecture and cell:cell interactions not possible on planar surfaces. Whether situated atop MEAs or engineered to contain optogenetic constructs, drug screening can be performed to assess brain organoid function and viability.
Three-dimensional peripheral nerve models. Peripheral nerve anatomy informs researchers to create 3D constructs that respect the compartmentalized and polar features found naturally. By containing soma either within restrictive chambers or growing them as spheroids, axon and glial outgrowth can be directed along MEA surfaces to assess electrophysiological function. Sensory neural constructs (top) can integrate a variety of neural subtypes and be used to study satellite glia in neuropathic pain models and the subsequent input into the dorsal horn. Motor neuron constructs (bottom) are currently being integrated into multiorgan NMJ models. Both systems are able to be myelinated by Schwann cells. Nerve fascicle formation, along with perineurial glia, can be studied.
Substrate and Mechanics
Recreating a physiologically relevant biochemical and biomechanical microenvironment is a key concern for any cell biologist when beginning an in vitro study. Extracellular matrix proteins are integral to creating biological niches, which promote the differentiation (Morante-Redolat and Porlan 2019) and maintenance of specific cell types, neurons, and glia in particular. Neural cell cultures have historically and still continue to be grown upon planar polymer and glass surfaces derivatized with extracellular matrix proteins, such as collagen IV, fibronectin, and laminin (Gordon et al. 2013). Laminins have proven to be of particular importance to neural development and functionality with laminin-111, the primary component of Matrigel (Hughes et al. 2010) and that produced by mouse Engelbreth-Holm-Swarm sarcoma cells, being the most used. While laminin-111 has proven a stalwart substrate, exploration of other isoforms, specifically those with the aphla-5 domain, has shown improved development of network formation (Hyysalo et al. 2017) and activity (Hyvarinen et al. 2019). Indeed, using a more targeted isoform could be of importance when attempting to recapitulate biological niches which have been shown to contain specific laminins in vivo (Nirwane and Yao 2018).
Mechanical characteristics of 2D or 3D platforms influence greatly cell density (Previtera et al. 2010), development (Barnes et al. 2017), and functionality (Barnes et al. 2017) with changes to material moduli helping to achieve either healthy homeostasis or to recapitulate disease environments (Wen et al. 2018). Studies have also sought to define critical stiffness parameters with 2D neurite outgrowth on thin films of methacrylate copolymers (Tuft et al. 2014), polydimethylsiloxane (Zhang et al. 2014), and polyacrylamide (Tanaka et al. 2018) consistently showing higher moduli promoting increased neurite outgrowth when grown on planar surfaces. Of note is the supraphysiological moduli employed within some studies that can be orders of magnitude above what is seen in vivo. Within physiological gradients, stiffness is still observed to affect network formation and outgrowth atop PDMS substrates (Lantoine et al. 2016). Interestingly, neurons continue to exhibit outgrowth on soft substrates (hundreds of Pascals) while astrocyte growth can be restricted (Georges et al. 2006). Avoiding the induction of possibly pathological states as seen in stiffer environments found in vivo such as a glial scar (Horner and Gage 2000) should therefore be considered when deciding a material’s modulus. Additional to material modulus, restricting embryonic stem cell (ESC)–derived brain organoids between two surfaces has provided some insight into the nascent stages of cortical folding. Controlling the material stiffness, growth compartment and possibly also hydrostatic pressure that is crucial to in vivo development may lead to more fully realized organoids with distinct cortical structures (Karzbrun et al. 2018).
Neuron/Glial Cell (Sub)types
Culturing of neuronal and glial cells has been extensively reviewed prior (Gordon et al. 2013; Belle et al. 2018). While many of these culture systems focus on non-human cultures, emerging protocols for the rapid generation of human (h) nervous systems from induced pluripotent stem cells (iPSC) is of particular interest to translatable MPS development. Tuning defined media formulations, incorporating inducible promoters and exploring 3D hiPSC differentiation have allowed for cellular differentiation timeline reductions from months to weeks, depending on the cell type. For example, oligodendrocytes can, via a doxycycline-inducible SOX10 promoter, be formed within roughly 20d (Garcia-Leon et al. 2018). Strategies requiring no genetic footprint require longer differentiation timescales with the best 3D development strategies taking 30wk (Madhavan et al. 2018) to form mature, myelinating oligodendrocytes. Similarly, hiPSCs can be differentiated into astrocytes in cortical spheroids using solely growth factor cocktails and be maintained for a staggering 590d in culture (Sloan et al. 2017). Many techniques work towards the end of improving differentiation timelines, especially with tightly defined culture regimens that accelerate differentiation of hiPSCs towards a neuronal progenitor lineage (Walsh et al. 2017). Further development of these rapid hiPSC differentiation strategies, especially those which result in co-cultures of neurons and glia, will be required in order to progress scalable MPSs for high-throughput screening applications.
Cell-Cell Interactions
Beyond characterizing the functionality of neurons, the ability to analyze interactions of neurons to glia is paramount to faithfully modelling in vivo processes, especially with the increasing appreciation for glia within neuropathologies such as Parkinson’s (Tremblay et al. 2019), Alzheimer’s (Y. S. Kim et al. 2018) and ischemic stroke (Hersh and Yang 2018). Powerful tools such as genetically encoded calcium reporters have allowed for the rapid analysis of signaling within astrocyte populations in response to neuronal activity (Savtchouk et al. 2018). Apart from rapid signaling, characterizing the metabolic outputs resulting from neuron:astrocyte interaction has been studied in 3D substrates, specifically the monitoring of glucose and acetate metabolism (Simao et al. 2016).
As oligodendrocytes appear to show preferential myelination of certain neuronal subtypes in vivo (Zonouzi et al. 2019), creating 3D models to study their cell-cell interactions with neurons is vital to rapidly assess development and response to injury. Furthermore, myelination is a key process for neuronal functionality and several human neurologic diseases have been associated with dysfunctional oligodendrocytes and myelin deficits, such as schizophrenia, multiple sclerosis (MS), amyotrophic lateral sclerosis (ALS) and periventricular leukomalacia (PVL) (Noseworthy et al. 2000; Billiards et al. 2008; Kang et al. 2013; Najjar and Pearlman 2015). Therefore, this feature becomes particularly important to include in MPS for drug development. However, oligodendrocyte differentiation in 3D has been assessed with exceptionally long in vitro culture times (up to 235d (Marton et al. 2019)), which may not be compatible with drug development strategies and timelines. This study was however able to show functional myelination and electrophysiological readouts sensitive to drugs blocking ionotropic glutamate receptors expressed by oligodendrocytes. Further, these oligodendrocyte spheroid cultures contained astrocytes and neurons and were able to track individual cell migration and mapped morphology and maturation via live imaging. Conversely, in a truly reductionist manner, models have been created which remove the neuron itself and opt for artificial axons to study oligodendrocyte myelination on 3D scaffolds (Espinosa-Hoyos et al. 2018), with ensheathment being promoted in laminin- and stiffness-dependent manners. Tuning cell-surface markers to help model myelination of specific neuron subclasses may even pave the way for further defining oligodendrocyte heterogeneity and subclasses, an underappreciated characteristic of a “functionally homogeneous” cell type (Marques et al. 2016).
Non-neuronal cells that serve barrier functions have been of interest when creating MPSs. The blood-brain barrier (BBB) is widely the most studied barrier and has been reviewed extensively prior (Banks 2016; Villabona-Rueda et al. 2019). 3D in vitro models of the BBB have been built to study Alzheimer’s disease (Shin et al. 2019), interactions between astrocytes and vascular cells (Campisi et al. 2018), and the modelling of metastatic brain tumors (Xu et al. 2016). Organoid models have even been created which produce cerebrospinal fluid from choroid plexus epithelia derived from human stem cells (Pellegrini et al. 2020). Notably, these choroid plexus organoids contained pertinent transporters integral to barrier functionality and also secreted a host of relevant proteins one would expect to find in cerebral spinal fluid (49/50 of the most detected proteins in the organoid model are also found within in vivo samples). And while not necessarily a barrier-forming cell, satellite glia within the periphery envelope dorsal root ganglion somata and perform a litany of functions related to pain processing (Costa and Moreira Neto 2015), sympathetic transmission and neuron survival (Enes et al. 2020). There is also evidence to suggest that satellite glia serve as a reservoir of arrested Schwann cells that, via regulation of cadherin-19, may leave the soma and become mature, myelinating cells should cues arise, such as in injury (George et al. 2018).
Histoarchitecture
Modeling any tissue with fidelity ultimately leads to consideration of 3D microarchitecture, as all tissues are inherently 3D. The aforementioned characteristics culminate to recapitulate niches found in neural tissue with the now added dimensionality of non-planar culture surfaces. For example, while the fasciculation or bundling of nerves is of importance to modeling the peripheral nerve, this would be an erroneous design criterion for the central nervous system where neuroanatomy adopts stratified architectures in regions such as the cortex or hippocampus. While organotypic slice cultures are still used to study the spinal cord (Musto et al. 2019) and brain, recent advances have seen central nervous system neurons grown on mechanically controlled anisotropic scaffolds (Kim et al. 2017), porous microlattices (S. Li et al. 2018) and 3D bioprinted substrates (Antill-O’Brien et al. 2019) in order to recreate aspects of 3D macrostructure or neural connectivity seen in the brain. The peripheral nerve has also seen advancements in growing explant or spheroid cultures within anisotropic materials (Anderson et al. 2018; George et al. 2019) and growth restrictive hydrogels (Huval et al. 2015; Nguyen et al. 2019; Sharma et al. 2019; Kramer et al. 2020), respectively. Ultimately, the inherent qualities of anatomical histoarchitecture should guide the design of microarchitectural features of microphysiological devices.
Functional Assessment
Functionality of a neuronal system is paramount for any meaningful data acquisition. Electrophysiology outcomes such as those acquired via microelectrode arrays (MEA) are the emerging gold standard for in vitro studies and multiple studies have highlighted the difference between 2D and 3D systems. Stiff, laminin-coated glass beads used as a 3D network positioned above an MEA showed variant burst rates and stimulus-evoked responses compared with 2D culture of e18 rat hippocampal neurons (Frega et al. 2014). Softer substrates, such as anisotropic alginate-based scaffolds (Anderson et al. 2018; George et al. 2019) or carbon nanotube-containing PDMS (Bosi et al. 2015), have also shown varied activity including capabilities of delineating capsaicin-dependent spikes of peripheral nerve culture and improving overall hippocampal neuron activity, respectively. With 3D systems outperforming 2D, future development should surround exploring further how 3D systems may affect arborization (Kayal et al. 2019), synaptic maturation and functional activity, especially in the presence of drugs.
Microscopy utilizing ratiometric fluorescent dyes, genetically encoded calcium reporters and two-photon imaging techniques has allowed for the real-time assessment of synchronous activity in many tissue types, including neurons (Grienberger and Konnerth 2012; Mitani and Komiyama 2018). Similarly, the utilization of optogenetic technology has been used in 3D neuronal models to induce action potentials selectively via specific genetic promoters and to activate organ systems through synapses (Renault et al. 2015; Lee et al. 2019), such as the neuromuscular junction (Osaki et al. 2020). Multiplanar imaging of complex neural circuits has also improved and allows for the study of neurons both in vitro and in vivo (Yang et al. 2016). Construct dimensionality influences this synchronous behavior as seen with hippocampal neurons grown upon planar graphene surfaces or embedded within 3D graphene foams (Ulloa Severino et al. 2016). By affecting the structural connectivity of the tissue (i.e., culturing with the foam), the functional connectivity was improved, resulting in a greater degree of high synchronous activity as compared to only moderate synchronous activity within the 2D controls.
Emerging Superiority of 3D Neural Cultures
Advancements in 3D culture techniques have presented researchers with two paradigms by which to approach experimental designs. While 2D cell culture configurations will continue to be useful for answering certain questions, it is becoming apparent that 3D culture configurations can best capture the complexity of the nervous system, whether considered by structural, functional, or effective connectivity, to provide the most clinically translatable outcomes. Properly replicating in vivo responses cannot be achieved in 2D environments as they do not provide necessary cell signaling cues for normal nervous system development (Alepee et al. 2014). Therefore, successfully generating these neuronal connections in vitro requires guided 3D tissue development and maturation (Lai et al. 2012) to best recapitulate what is observed in vivo. Improved cell:cell interactions and signaling lead to complex structure formation such as the 4-part synapse including the presynaptic and post-synaptic neurons, the astrocyte, and the microglia (Schafer et al. 2013). It has been shown that the activity better mimics what is seen in vivo when cells are grown in a 3D environment. Cells in the brain develop in a 3D space, and these multicellular complex networks that are formed are important for greater neural function (Frega et al. 2014). Comparable networks can have drastically different effects on network bursting and synchrony when cultured in 3D, which may further reveal cellular responses that further mimic in vivo responses.
Modelling the Central Nervous System
The human central nervous system is a complex network of various neuronal, glial and immune cells, which can be divided into discrete architectural areas that define motor, sensory, memory or other functions. By this classical approach, the brain can be assessed by a structural connectivity approach wherein defined areas can be classified neuroanatomically via long-standing neuroimaging techniques. Further characterizing these structures based upon functional readouts (e.g., electrophysiology, fMRI, PET) can show functional connectivity wherein “an observable phenomenon… can be quantified with measures of statistical dependencies” (Friston 2011). This allows for observations of connectivity between brain centers not dependent upon specific experimental models or hypothesis. Experimental approaches lend themselves to what is termed effective connectivity which “corresponds to the parameter of a model that tries to explain observed dependencies” (Friston 2011). With these definitions in mind, the approach to building meaningful platforms to study the CNS should create a functionally connected network of tissue which can be assessed for effective connectivity through experimentation and physiologically relevant outcomes (Fig. 1). As individual in vitro studies will undoubtably require various functional outcomes, the desired structural parameters of the chosen model will be largely dependent on the specific questions being asked.
Microengineered Culture Systems
Efforts to use microfabrication techniques to control the organization and connectivity of CNS cells in vitro date back at least several decades to the invention of the eponymous Campenot chamber by Robert Campenot (1977) (Campenot 1977). These culture systems typically make use of relatively simple structural features such as microtunnels and microfluidic channels to organize cells, promote specified connectivity, and enable compartmentalization, often combined with microelectrode arrays for physiological assessment (Fig. 3). Advancements in approaches such as these have been reviewed in detail recently (Nikolakopoulou et al. 2020). While typically restricted to 2D formats, these model systems excel especially when it is important to compartmentalize cell types or segregate axons from somas so that drugs or neuromodulators may be applied to one compartment at a time.
MPS for modeling CNS interconnected functionality has been achieved with engineered culture systems using microfabricated chambers for specifying synaptic connectivity and microelectrodes for evaluating function. Adapted from Moutaux et al. (2018), with permission from The Royal Society of Chemistry.
Brain Organoid Models
Brain organoids are made by differentiating hiPSCs or embryonic stem cells through a neural lineage to generate the major cells that make up the human brain (Lancaster et al. 2013). These models generally include relevant mature cell types of neurons are capable of self-assembly into organoids from distinct brain regions (Fig. 4). Diversity in incorporated cell types and culture conditions yield varying, complex organoids that are capable of being engineered to enable specific neuron:glia compositions and interactions, namely myelination. (Fig. 5). The major neuronal subtypes studied include glutamatergic, GABAergic, dopaminergic and serotonergic neurons. Importantly, cell numbers can be analyzed to study the effect of specific drugs on each cell population and the effect that other cells in the system may be having on each other. Normal development of these cells gives rise to important microstructures of the brain including neuronal axons and dendrites. In this way, synaptic communication can be studied and between excitatory and inhibitory neurons, leading to a deeper understanding of brain circuitry.
Brain organoids rely on self-assembly during differentiation from pluripotent cells. Adjusting culture conditions has produced large organoids exhibiting self-organized internal structure as well as organoids representing distinct brain regions. Adapted from Qian et al. (2016), with permission from Elsevier.
Brain spheroids for the 3D modeling of the central nervous system. Developed spheroids show a diverse population of both (a) neuronal and (b) glial cells throughout the bulk of the organoid. (c) Myelination is also readily apparent and the progression of myelination within the spheroid can be assessed via both (d) immunohistochemistry and (e) TEM. Reproduced from Pamies et al. (2017), under Creative Commons license.
These models benefit by allowing for the physiological study of neuronal electrical activity. By plating these on MEA plates, the spontaneous electrical activity can be measured in a high-throughput format. Electrical characteristics can be measured when cells are exposed to chemicals or even exogenous electrical stimuli. The multielectrode arrays also allow for the study of network activity, which is related to more complex cellular interactions. One study has demonstrated the detection of complex oscillatory waves from cortical organoids maturing for long periods in culture. Remarkably, when followed for several months, dynamic oscillatory waves began to give rise to network synchrony that exhibited phase-amplitude coupling (Trujillo et al. 2019). Such advanced functional metrics as these may serve to model complex neurological phenomena in vitro that may have implications in disorders such as epilepsy, autism and mental illness.
Brain Spheroid Myelination
Oligodendrocyte differentiation leads to myelination in brain spheroid models, but many models currently being used do not include this cell type. Oligodendrocyte differentiation from iPSCs has been difficult and only a few organoid models currently include these cell types. One study demonstrated the formation of brain spheroids that include oligodendrocytes and endogenous myelination (Pamies et al. 2017) (Fig. 5c–e). Co-localization of neuronal neurofilament-heavy protein (NF-H) with MBP immunostaining suggested evidence of myelin formation as early as 4wk into differentiation and up to 40% myelinated axons by 8wk of development. Transmission electron microscopy confirmed the presence of compact myelin microstructure by 8wk of development and enabled measurement of G ratios, which may serve as a metric for modeling myelin diseases. This approach may lend itself for use in studying demyelination and remyelination. Cuprizone and lysophosphatidylcholine (LPC) treatment (Vereyken et al. 2009) is commonly used to induce demyelination in the CNS in animal models (Zhan et al. 2020). With this drop in myelination, this system can also be used for remyelination assays, which would be especially important for studying relapse-remitting multiple sclerosis. The causes for remyelination failure in more progressive phases of multiple sclerosis are unknown (Charles et al. 2002) and shows that this could be a powerful tool for these studies.
Modeling Peripheral Nerve
The peripheral nervous system serves to relay central outputs to target tissues and as the sensory input responsible for gathering normal and noxious stimuli from the extremities and transmitting it to the dorsal horn of the spinal cord. The development of in vitro models of peripheral nerve has been reviewed in detail recently (Pollard et al. 2019). Given the unique pseudo unipolar nature of neurons within the dorsal root ganglia, the collection of somas stemming from the dorsal ramus, creating MPSs which are able to recapitulate the distinct anatomical confinement of peripheral nerves is preeminent to design consideration (Fig. 2). Herein, we will discuss these important structural elements, the ability to integrate them with microelectrode platforms and how to approach greater physiological relevance by inducing myelination and assessing drug cytotoxicity.
Peripheral Nerve Histoarchitecture
As noted above, the peripheral nervous system is composed of neurons which have either a unipolar (motor) morphology with a single axon extending from the soma in the spinal cord to the periphery or else a pseudo-bipolar (sensory) morphology that branches towards the periphery and the dorsal horn of the spinal cord. Merely plating peripheral neurons on planar surfaces does not allow for recreation of this morphology; therefore, a myriad of engineering techniques have been employed to generate MPSs that can appropriately induce polarized and parallel-aligned axon growth mimicking nerve structure. 3D printed microchannels have been able to guide DRG axon outgrowth and co-culture with Schwann cells has shown successful induction of myelination and Schwann cell proliferation (Johnson et al. 2016; Sakai et al. 2017) (Fig. 6). A rudimentary perineurium has also been achieved within alginate constructs with open capillaries. Coating of these constructs with laminin allowed for nerve outgrowth and enough of a polarizing signal to form perineurial cells marked by tight junction expression and distinct localization around the nerve bundle (Anderson et al. 2018).
Peripheral nervous system compartmentalization through 3D printed constructs. (a) Schematic and formed 3D printed chamber for compartmentalized cell growth. From left to right, these three chambers contain (b) neuron cell bodies, (c) Schwann cells growing along axonal projections, and (d) axon terminals. Models such as this one can ensure compartmentalized growth and can realize aligned growth of various cell types. Adapted from Johnson et al. (2016), with permission from The Royal Society of Chemistry.
MEA Recording
In vitro recordings of neurons on the microscale have long been accomplished using patch clamp. However, this method allows only for single cell analysis, which does not serve to capture the organ level activity created by 3D MPSs. Electro-optical methods such as channel rhodopsin and calcium signaling imaging such as genetically encoded fluorescent reporters have helped push recordings towards multicell systems, but the microelectrode array remains the gold standard in local field recordings of neural tissue constructs. This technology reviewed by Obien et al. (2014) combines tissue on a chip engineering designs with real-time electrical outputs necessary to quantifying neural processes. Of special interest is combining developed 3D culture systems with these traditionally planar recording modalities; progress has been made previously in 2D to this end and has been achieved with compartmentalization, polarization, and tissue alignment (Fig. 7). Bridging this gap would allow for the real-time recording of peripheral nerve organoid cultures over long experimental time courses instead of using manually inserted field electrodes which can damage tissue and introduce contamination.
Peripheral nerve MPS demonstrating compartmentalization, polarization, and alignment in 2D, along with integration of microelectrodes for spike sorting and observation of axonal conduction. Adapted from Sakai et al. (2017), by permission of Oxford University Press.
3D Peripheral Nerve MPS
Peripheral nerve tissue-on-a-chip platforms should build upon microfabrication techniques and tune them towards the development of testable 3D models with physiologically relevant outcomes, notably electrophysiology. These constructs should be able to direct axonal growth, support glial (e.g., myelination) and neuronal (e.g., synaptic transmission) cell functions and be able to record electrophysiological signals with high signal-to-noise ratios. As within any cellular system, maintaining high viability is crucial. Thick tissue sections and large organoids lack vasculature and therefore do not have the ability to supply oxygen deep into tissue. To overcome the formation of necrotic cores, it is then important to maintain a tissue volume that does not exceed a radius or depth of roughly 200μm (i.e., exceeding the passive diffusion distance of oxygen within tissue (Rouwkema et al. 2010)). One solution to this problem is the formation of defined spheroids with tunable sizes based upon cell numbers. These spheroids, which exhibit excellent viability (Fig. 8a), are able to be manually manipulated and placed within tissue constructs composed of various substrates, such as methacrylated gelatins or more ubiquitous ECM scaffolds such as Matrigel. Notably, these spheroids can be cultured for many weeks without losing functionality. These long-term culture conditions allow for extensive neurite outgrowth in all dimensions with growth-restrictive hydrogels being able to confine dense axon beds to specific areas for electrophysiological recordings. These constructs can then be used for electrophysiological assessment and exhibit peaks that are able to be defined by treatment with drugs (Kramer et al. 2020). Additional to neuron function, glia are able to grow and myelinate, more on that next.
Peripheral nerve modeling and myelination. (a) Rat primary sensory neuron tissue grown as a spheroid within a growth-restrictive hydrogel can yield (b) induced compound action potentials detected by extracellular field recording. (c, d) Similar spheroids composed of iPSC-derived human neurons can show outgrowth and myelination that can be confirmed by (e) TEM micrographs showing compact myelin rings. Reproduced from Sharma et al. (2019), under Creative Commons license.
Induction of Myelination
Primary embryonic rat tissue has long served as the basis for peripheral nerve studies and has elucidated mechanisms of myelination by Schwann cells dating back to the 1980s (Eldridge et al. 1987). However, myelination of human axons by human Schwann cells in vitro has been much more elusive (Monje 2020). These myelination studies have long existed within 2D systems such as those described above wherein cells are cultured atop ECM coated glass coverslips without any discrete compartmentalization. While some microfluidic chambers and 3D biomaterial constructs have incrementally moved away from this by promoting alignment via construct architecture, generating a dense, aligned 3D nerve with myelination has only recently been achieved with human cells (Sharma et al. 2019). Growth restrictive 3D hydrogels have shown capable in generating myelinated human MPSs by combining commercially available motor neuron cultures with Schwann cells. Addition of ascorbic acid to optimized co-seeding densities was able to induce myelination as observed via immunofluorescence and TEM (Fig. 8c–e). Compound action potentials were able to be recorded with conduction velocity and G ratios able to be quantified. Further development of this system is geared towards the fabrication of peripheral sensory neuronal systems with the promotion of Schwann cell migration being a critical goal to enable myelination of greater length scales.
Translational Aspects of MPS: Modelling Neuropathies
Given the aforementioned structural and functional benefits of modelling the development and physiology of neural tissues in 3D, it would be reasonable to conclude that studying pathologies within 3D systems is beneficial over models that utilize planar substrates. Organoids in particular provide robust environments to assess both peripheral and central nervous system diseases. The effects of cell proliferation, migration, genetic anomalies, and environmental factors on organoid development and function have been previously reviewed in the context of neuropsychiatric disorders (Amin and Pasca 2018). This has pushed the field of neuroscience and ultimately patient care forward by allowing for basic science approaches to future pathophysiology studies and drug development that do not rely on either post-mortem assessment of tissue or non-invasive techniques (such as MRI, PET, CT) that lack the resolution required to observe ultra-fine structures (e.g., dendritic spines). Here, we will highlight specific areas what have seen advancements of bridging the development of MPS to the translational study of neuropathies, i.e., moving from microphysiological to micropathophysiological systems.
Connection to Target Organs: Modelling the Neuromuscular Junction
Peripheral nerve models on their own (i.e., motor or sensory neurons individually or in combination with glia) hold the capacity to elucidate many basic science inquiries and are entering nascent stages of allowing for higher throughput capacity for drug screening. By integrating these neural MPSs with the target tissue, their innervation of or providing reception for in vivo tissues opens up new avenues of research focusing on pathologies that affect both the peripheral nervous system and said target tissues. The most widely studied target organ structure to-date remains the neuromuscular junction (Omar et al. 2020) (NMJ), primarily that of skeletal muscle to study diseases such as Amyotrophic Lateral Sclerosis (ALS), myasthenia gravis (Faustino Martins et al. 2020) and Duchenne muscular dystrophy (Afshar Bakooshli et al. 2019). While advanced 2D culture chambers are still being utilized to screen drugs in vitro (Santhanam et al. 2018), research has begun turning to 3D co-cultures of spinal neurons and skeletal muscles fibers, whether in organoid formats encapsulated within fibrinogen (Afshar Bakooshli et al. 2019) or silk fibroin hydrogels (Dixon et al. 2018). Importantly, these systems showed functional NMJ formation, measured skeletal fiber contractility, modeled axonal transport of pathophysiological TAR DNA binding protein 43 (TDP-43) in the case of ALS and could even induce a model of myasthenia gravis with patient-derived antibodies targeting NMJs. Innervation of muscular fibers was also able to be sped up in 3D versus 2D cultures as well as more robust fiber formation as defined by contractility, hypertrophy, calcium handling and expression of key receptors, notably the acetylcholine receptor.
Brain Spheroid Neurotoxicity Assay
Brain spheroids have been shown to be effective models for studying neurotoxicity. Treatment with rotenone (Nzou et al. 2018) has led to evidence of neuronal toxicity in vitro (Pamies et al. 2018). This toxicity was higher in spheroids that were treated earlier in development when compared with spheroids treated at later time points. Dopaminergic neurons were especially sensitive to rotenone treatment and showed selective toxicity at “non-cytotoxic” concentrations, sparing astrocytes and other neuron subtypes. Moreover, the model identified the antidepressant paroxetine as a developmental neurotoxicant where synaptogenesis, neurite outgrowth and the number of oligodendrocyte were significantly decreased at therapeutic blood concentrations (Zhong et al. 2020). This demonstrates that this model’s usefulness for studying neurotoxicity (Schmidt et al. 2017) and developmental neurotoxicity (Smirnova et al. 2014). The use of iPSC also allows for assessment of gene-environmental interactions as donors with different genetic backgrounds can be used.
Neuroinflammation of the Central Nervous System
The brain spheroid models discussed so far have been composed of cells that arise from the neuroectoderm that make up the CNS, but do not include microglia, another vital cell found in the CNS for normal brain development and function. Microglia are the resident phagocytic immune cells in the brain and spinal cord.
By adding microglia to these spheroid models, neuroinflammation can be studied (Abreu et al. 2018). LPS treatment is well documented to cause neuroinflammation and neurotoxicity and is a known stimulator of microglia. Treatment of brain spheroids containing microglia have shown similar responses to what is seen in vivo. Treatment with LPS causes an increase in the expression of inflammatory cytokines. An inflammatory response was also detected in response to flavivirus infection shown in the experiment. This demonstrates the ability of this model to be used in further research of dengue and Zika viruses and how the brain spheroid model also does well for studying neuroinflammation. Finally, the relevance of studying viral infection, replication and spread within the nervous system cannot be diminished, especially due to the recent SARS-CoV-2 pandemic. Brain spheroid models have helped elucidate these key facets of viral tropism within the CNS, and the continued study in vitro will help to model the currently unknown long-term effects of this increasingly ubiquitous pathogen (Bullen et al. 2020).
Cancer
Brain MPS have also enabled cancer studies by grafting or inducing for example glioblastoma in brain organotypic cultures. Glioblastoma, one of the most aggressive brain tumors, was introduced into the 3D human stem cell–derived models via genetic editing or by co-culturing with glioblastoma cells (Nayernia et al. 2013; Bian et al. 2018; Ogawa et al. 2018; Linkous et al. 2019; Plummer et al. 2019; Krieger et al. 2020). Such cancer-bearing organoids promise personalized medicine by exploring most effective therapeutics for a given patient as well as the development of novel therapeutics (Plummer et al. 2019). Culturing the cancer in a physiological environment and not just as cancer organoids promises to recreate the cancer microenvironment, which is critically impacting on tumor behavior. The approach also allows to directly compare the drug effects on cancer and healthy tissue, defining therapeutic windows.
Conclusion and Future Perspectives
Microphysiological systems combine facets of bioengineering, molecular biology, material science and computer modeling to best recapitulate natural processes in defined, testable systems. Neural MPSs, for both the peripheral and central nervous systems, have allowed for basic science research in neuroscience to be propelled beyond traditional 2D culture and the integration of 3D culture methods has only furthered neural MPSs. While much progress has been made, opportunities remain in ways to best recreate cytoarchitectures and organ-level anatomy, all while focusing on translatable outcomes such as overcoming challenges in the drug discovery pipeline. Further incorporation of glia, especially satellite glia in the peripheral and microglia in the central nervous system, will expand cell:cell interaction studies to how understudied cell types affect normal and pathophysiological states. The advancement of bioengineered tissue morphogenesis (Fedorchak et al. 2020), formation of “assembloids” from region-specific organoids (Xiang et al. 2017), and axonal connection of multiple tissues representing the peripheral and central nervous systems (Bowser and Moore 2019) will allow for modeling of distinct neuroanatomical features separated by fibers, akin to how these structures appear in vivo. This guided cross-talk of distinct organoids of physiologically relevant tissue will ideally be ever-expanded upon moving forward.
Carrying these ideas forward to different distinct loci within the brain, such as the substantia nigra to the putamen, will help elucidate complex pathologies that arise from defects of deep brain structures. Better recreating other distinct anatomical features (e.g., the perineurium in the peripheral and the blood-brain barrier in the central nervous system) enables 3D systems to study how drugs and pathogens cross these barriers and affect neural and glial tissue alike. Finally, being able to faithfully record electrophysiological outcomes must move away from planar electrodes found on MEAs and not rely on tissue-disruptive field recordings. While the automation of patch clamping via robotics (Holst et al. 2019) expediates a normally time and labor-intensive process (Milligan et al. 2009), integrated nanoelectronic electrodes within tissues may very well be the future (Q. Li et al. 2019). Developing 3D, integrated electrodes into neural MPSs will allow for long-term, real-time monitoring of spontaneous and inducible activity of cultured tissue (Soscia et al. 2020) and provide for more rapid testing of pharmaceuticals within these constructs. Combining the complicated architectures of 3D MPS with appropriate functional aspects to truly create an in vivo mimic will be the key for these systems not only to become major tools for drug screening applications and neurological discoveries but also to replace animal testing completely.
References
Abreu CM, Gama L, Krasemann S, Chesnut M, Odwin-Dacosta S, Hogberg HT, Hartung T, Pamies D (2018) Microglia increase inflammatory responses in iPSC-derived human BrainSpheres. Front Microbiol 9:2766. https://doi.org/10.3389/fmicb.2018.02766
Afshar Bakooshli M, Lippmann ES, Mulcahy B, Iyer N, Nguyen CT, Tung K et al (2019) A 3D culture model of innervated human skeletal muscle enables studies of the adult neuromuscular junction. Elife 8:e44530. https://doi.org/10.7554/eLife.44530
Alepee N, Bahinski A, Daneshian M, De Wever B, Fritsche E, Goldberg A et al (2014) State-of-the-art of 3D cultures (organs-on-a-chip) in safety testing and pathophysiology. ALTEX 31(4):441–477. https://doi.org/10.14573/altex.1406111
Amin ND, Pasca SP (2018) Building models of brain disorders with three-dimensional organoids. Neuron 100(2):389–405. https://doi.org/10.1016/j.neuron.2018.10.007
Anderson WA, Willenberg AR, Bosak AJ, Willenberg BJ, Lambert S (2018) Use of a capillary alginate gel (Capgel) to study the three-dimensional development of sensory nerves reveals the formation of a rudimentary perineurium. J Neurosci Methods 305:46–53. https://doi.org/10.1016/j.jneumeth.2018.05.003
Antill-O’Brien N, Bourke J, O’Connell CD (2019) Layer-by-layer: the case for 3D bioprinting neurons to create patient-specific epilepsy models. Materials (Basel) 12(19). https://doi.org/10.3390/ma12193218
Banks WA (2016) From blood-brain barrier to blood-brain interface: new opportunities for CNS drug delivery. Nat Rev Drug Discov 15(4):275–292. https://doi.org/10.1038/nrd.2015.21
Barnes JM, Przybyla L, Weaver VM (2017) Tissue mechanics regulate brain development, homeostasis and disease. J Cell Sci 130(1):71–82. https://doi.org/10.1242/jcs.191742
Belle AM, Enright HA, Sales AP, Kulp K, Osburn J, Kuhn EA, Fischer NO, Wheeler EK (2018) Evaluation of in vitro neuronal platforms as surrogates for in vivo whole brain systems. Sci Rep 8(1):10820. https://doi.org/10.1038/s41598-018-28950-5
Bian S, Repic M, Guo Z, Kavirayani A, Burkard T, Bagley JA, Krauditsch C, Knoblich JA (2018) Genetically engineered cerebral organoids model brain tumor formation. Nat Methods 15(8):631–639. https://doi.org/10.1038/s41592-018-0070-7
Billiards SS, Haynes RL, Folkerth RD, Borenstein NS, Trachtenberg FL, Rowitch DH, Ligon KL, Volpe JJ, Kinney HC (2008) Myelin abnormalities without oligodendrocyte loss in periventricular leukomalacia. Brain Pathol 18(2):153–163. https://doi.org/10.1111/j.1750-3639.2007.00107.x
Bosi S, Rauti R, Laishram J, Turco A, Lonardoni D, Nieus T, Prato M, Scaini D, Ballerini L (2015) From 2D to 3D: novel nanostructured scaffolds to investigate signalling in reconstructed neuronal networks. Sci Rep 5:9562. https://doi.org/10.1038/srep09562
Bowser DA, Moore MJ (2019) Biofabrication of neural microphysiological systems using magnetic spheroid bioprinting. Biofabrication 12(1):015002. https://doi.org/10.1088/1758-5090/ab41b4
Bullen CK, Hogberg HT, Bahadirli-Talbott A, Bishai WR, Hartung T, Keuthan C et al (2020) Infectability of human BrainSphere neurons suggests neurotropism of SARS-CoV-2. ALTEX. https://doi.org/10.14573/altex.2006111
Campenot RB (1977) Local control of neurite development by nerve growth factor. Proc Natl Acad Sci U S A 74(10):4516–4519. https://doi.org/10.1073/pnas.74.10.4516
Campisi M, Shin Y, Osaki T, Hajal C, Chiono V, Kamm RD (2018) 3D self-organized microvascular model of the human blood-brain barrier with endothelial cells, pericytes and astrocytes. Biomaterials 180:117–129. https://doi.org/10.1016/j.biomaterials.2018.07.014
Centers for Disease Control (2020). New data show significant changes in drug overdose deaths, Atlanta, GA. Retrieved from https://www.cdc.gov/media/releases/2020/p0318-data-show-changes-overdose-deaths.html. Accessed 26 Oct 2020
Charles P, Reynolds R, Seilhean D, Rougon G, Aigrot MS, Niezgoda A et al (2002) Re-expression of PSA-NCAM by demyelinated axons: an inhibitor of remyelination in multiple sclerosis? Brain 125(Pt 9):1972–1979. https://doi.org/10.1093/brain/awf216
Costa FA, Moreira Neto FL (2015) Satellite glial cells in sensory ganglia: its role in pain. Rev Bras Anestesiol 65(1):73–81. https://doi.org/10.1016/j.bjan.2013.07.013
Dixon TA, Cohen E, Cairns DM, Rodriguez M, Mathews J, Jose RR, Kaplan DL (2018) Bioinspired three-dimensional human neuromuscular junction development in suspended hydrogel arrays. Tissue Eng Part C Methods 24(6):346–359. https://doi.org/10.1089/ten.TEC.2018.0062
Dowden H, Munro J (2019) Trends in clinical success rates and therapeutic focus. Nat Rev Drug Discov 18(7):495–496. https://doi.org/10.1038/d41573-019-00074-z
Eldridge CF, Bunge MB, Bunge RP, Wood PM (1987) Differentiation of axon-related Schwann cells in vitro. I. Ascorbic acid regulates basal lamina assembly and myelin formation. J Cell Biol 105(2):1023–1034. https://doi.org/10.1083/jcb.105.2.1023
Enes J, Haburcak M, Sona S, Gerard N, Mitchell AC, Fu W, Birren SJ (2020) Satellite glial cells modulate cholinergic transmission between sympathetic neurons. PLoS One 15(2):e0218643. https://doi.org/10.1371/journal.pone.0218643
Environmental Protection Agency (2015). Neurodevelopmental disorders. Retrieved from https://www.epa.gov/sites/production/files/2015-10/documents/ace3_neurodevelopmental.pdf. Accessed 26 Oct 2020
Espinosa-Hoyos D, Jagielska A, Homan KA, Du H, Busbee T, Anderson DG et al (2018) Engineered 3D-printed artificial axons. Sci Rep 8(1):478. https://doi.org/10.1038/s41598-017-18744-6
Faustino Martins JM, Fischer C, Urzi A, Vidal R, Kunz S, Ruffault PL, Kabuss L, Hube I, Gazzerro E, Birchmeier C, Spuler S, Sauer S, Gouti M (2020) Self-organizing 3D human trunk neuromuscular organoids. Cell Stem Cell 26(2):172–186 e176. https://doi.org/10.1016/j.stem.2019.12.007
Fedorchak NJ, Iyer N, Ashton RS (2020) Bioengineering tissue morphogenesis and function in human neural organoids. Semin Cell Dev Biol. https://doi.org/10.1016/j.semcdb.2020.05.025
Feigin VL, Vos T (2019) Global burden of neurological disorders: from global burden of disease estimates to actions. Neuroepidemiology 52(1-2):1–2. https://doi.org/10.1159/000495197
Frega M, Tedesco M, Massobrio P, Pesce M, Martinoia S (2014) Network dynamics of 3D engineered neuronal cultures: a new experimental model for in-vitro electrophysiology. Sci Rep 4:5489. https://doi.org/10.1038/srep05489
Friston KJ (2011) Functional and effective connectivity: a review. Brain Connect 1(1):13–36. https://doi.org/10.1089/brain.2011.0008
Garcia-Leon JA, Kumar M, Boon R, Chau D, One J, Wolfs E et al (2018) SOX10 single transcription factor-based fast and efficient generation of oligodendrocytes from human pluripotent stem cells. Stem Cell Rep 10(2):655–672. https://doi.org/10.1016/j.stemcr.2017.12.014
George D, Ahrens P, Lambert S (2018) Satellite glial cells represent a population of developmentally arrested Schwann cells. Glia 66(7):1496–1506. https://doi.org/10.1002/glia.23320
George DS, Anderson WA, Sommerhage F, Willenberg AR, Hines RB, Bosak AJ, Willenberg BJ, Lambert S (2019) Bundling of axons through a capillary alginate gel enhances the detection of axonal action potentials using microelectrode arrays. J Tissue Eng Regen Med 13(3):385–395. https://doi.org/10.1002/term.2793
Georges PC, Miller WJ, Meaney DF, Sawyer ES, Janmey PA (2006) Matrices with compliance comparable to that of brain tissue select neuronal over glial growth in mixed cortical cultures. Biophys J 90(8):3012–3018. https://doi.org/10.1529/biophysj.105.073114
Gordon J, Amini S, White MK (2013) General overview of neuronal cell culture. Methods Mol Biol 1078:1–8. https://doi.org/10.1007/978-1-62703-640-5_1
Grienberger C, Konnerth A (2012) Imaging calcium in neurons. Neuron 73(5):862–885. https://doi.org/10.1016/j.neuron.2012.02.011
Hersh J, Yang SH (2018) Glia-immune interactions post-ischemic stroke and potential therapies. Exp Biol Med (Maywood) 243(17-18):1302–1312. https://doi.org/10.1177/1535370218818172
Holst GL, Stoy W, Yang B, Kolb I, Kodandaramaiah SB, Li L, Knoblich U, Zeng H, Haider B, Boyden ES, Forest CR (2019) Autonomous patch-clamp robot for functional characterization of neurons in vivo: development and application to mouse visual cortex. J Neurophysiol 121(6):2341–2357. https://doi.org/10.1152/jn.00738.2018
Horner PJ, Gage FH (2000) Regenerating the damaged central nervous system. Nature 407(6807):963–970. https://doi.org/10.1038/35039559
Hughes CS, Postovit LM, Lajoie GA (2010) Matrigel: a complex protein mixture required for optimal growth of cell culture. Proteomics 10(9):1886–1890. https://doi.org/10.1002/pmic.200900758
Huval RM, Miller OH, Curley JL, Fan Y, Hall BJ, Moore MJ (2015) Microengineered peripheral nerve-on-a-chip for preclinical physiological testing. Lab Chip 15(10):2221–2232. https://doi.org/10.1039/c4lc01513d
Hyvarinen T, Hyysalo A, Kapucu FE, Aarnos L, Vinogradov A, Eglen SJ et al (2019) Functional characterization of human pluripotent stem cell-derived cortical networks differentiated on laminin-521 substrate: comparison to rat cortical cultures. Sci Rep 9(1):17125. https://doi.org/10.1038/s41598-019-53647-8
Hyysalo A, Ristola M, Makinen ME, Hayrynen S, Nykter M, Narkilahti S (2017) Laminin alpha5 substrates promote survival, network formation and functional development of human pluripotent stem cell-derived neurons in vitro. Stem Cell Res 24:118–127. https://doi.org/10.1016/j.scr.2017.09.002
Johnson BN, Lancaster KZ, Hogue IB, Meng F, Kong YL, Enquist LW, McAlpine MC (2016) 3D printed nervous system on a chip. Lab Chip 16(8):1393–1400. https://doi.org/10.1039/c5lc01270h
Kang SH, Li Y, Fukaya M, Lorenzini I, Cleveland DW, Ostrow LW, Rothstein JD, Bergles DE (2013) Degeneration and impaired regeneration of gray matter oligodendrocytes in amyotrophic lateral sclerosis. Nat Neurosci 16(5):571–579. https://doi.org/10.1038/nn.3357
Karzbrun E, Kshirsagar A, Cohen SR, Hanna JH, Reiner O (2018) Human brain organoids on a chip reveal the physics of folding. Nat Phys 14(5):515–522. https://doi.org/10.1038/s41567-018-0046-7
Kayal C, Moeendarbary E, Shipley RJ, Phillips JB (2019) Mechanical response of neural cells to physiologically relevant stiffness gradients. Adv Healthc Mater 9:e1901036. https://doi.org/10.1002/adhm.201901036
Kim SH, Im SK, Oh SJ, Jeong S, Yoon ES, Lee CJ, Choi N, Hur EM (2017) Anisotropically organized three-dimensional culture platform for reconstruction of a hippocampal neural network. Nat Commun 8:14346. https://doi.org/10.1038/ncomms14346
Kim YS, Jung HM, Yoon BE (2018) Exploring glia to better understand Alzheimer’s disease. Anim Cells Syst (Seoul) 22(4):213–218. https://doi.org/10.1080/19768354.2018.1508498
Kramer L, Nguyen HT, Jacobs E, McCoy L, Curley JL, Sharma AD, Moore MJ (2020) Modeling chemotherapy-induced peripheral neuropathy using a nerve-on-a-chip microphysiological system. ALTEX. https://doi.org/10.14573/altex.2001181
Krieger TG, Tirier SM, Park J, Jechow K, Eisemann T, Peterziel H, Angel P, Eils R, Conrad C (2020) Modeling glioblastoma invasion using human brain organoids and single-cell transcriptomics. Neuro-Oncology 22:1138–1149. https://doi.org/10.1093/neuonc/noaa091
Lai Y, Cheng K, Kisaalita W (2012) Three dimensional neuronal cell cultures more accurately model voltage gated calcium channel functionality in freshly dissected nerve tissue. PLoS One 7(9):e45074. https://doi.org/10.1371/journal.pone.0045074
Lancaster MA, Renner M, Martin CA, Wenzel D, Bicknell LS, Hurles ME, Homfray T, Penninger JM, Jackson AP, Knoblich JA (2013) Cerebral organoids model human brain development and microcephaly. Nature 501(7467):373–379. https://doi.org/10.1038/nature12517
Lantoine J, Grevesse T, Villers A, Delhaye G, Mestdagh C, Versaevel M, Mohammed D, Bruyère C, Alaimo L, Lacour SP, Ris L, Gabriele S (2016) Matrix stiffness modulates formation and activity of neuronal networks of controlled architectures. Biomaterials 89:14–24. https://doi.org/10.1016/j.biomaterials.2016.02.041
Lee SY, George JH, Nagel DA, Ye H, Kueberuwa G, Seymour LW (2019) Optogenetic control of iPS cell-derived neurons in 2D and 3D culture systems using channelrhodopsin-2 expression driven by the synapsin-1 and calcium-calmodulin kinase II promoters. J Tissue Eng Regen Med 13(3):369–384. https://doi.org/10.1002/term.2786
Leslie DL, Ba DM, Agbese E, Xing X, Liu G (2019) The economic burden of the opioid epidemic on states: the case of Medicaid. Am J Manag Care 25(13 Suppl):S243–S249. Retrieved from https://www.ncbi.nlm.nih.gov/pubmed/31361426. Accessed 26 Oct 2020
Li Q, Nan K, Le Floch P, Lin Z, Sheng H, Blum TS, Liu J (2019) Cyborg organoids: implantation of nanoelectronics via organogenesis for tissue-wide electrophysiology. Nano Lett 19(8):5781–5789. https://doi.org/10.1021/acs.nanolett.9b02512
Li S, Severino FPU, Ban J, Wang L, Pinato G, Torre V, Chen Y (2018) Improved neuron culture using scaffolds made of three-dimensional PDMS micro-lattices. Biomed Mater 13(3):034105. https://doi.org/10.1088/1748-605X/aaa777
Linkous A, Balamatsias D, Snuderl M, Edwards L, Miyaguchi K, Milner T, Reich B, Cohen-Gould L, Storaska A, Nakayama Y, Schenkein E, Singhania R, Cirigliano S, Magdeldin T, Lin Y, Nanjangud G, Chadalavada K, Pisapia D, Liston C, Fine HA (2019) Modeling patient-derived glioblastoma with cerebral organoids. Cell Rep 26(12):3203–3211 e3205. https://doi.org/10.1016/j.celrep.2019.02.063
Madhavan M, Nevin ZS, Shick HE, Garrison E, Clarkson-Paredes C, Karl M, Clayton BLL, Factor DC, Allan KC, Barbar L, Jain T, Douvaras P, Fossati V, Miller RH, Tesar PJ (2018) Induction of myelinating oligodendrocytes in human cortical spheroids. Nat Methods 15(9):700–706. https://doi.org/10.1038/s41592-018-0081-4
Marques S, Zeisel A, Codeluppi S, van Bruggen D, Mendanha Falcao A, Xiao L, Li H, Haring M, Hochgerner H, Romanov RA, Gyllborg D, Munoz-Manchado AB, la Manno G, Lonnerberg P, Floriddia EM, Rezayee F, Ernfors P, Arenas E, Hjerling-Leffler J, Harkany T, Richardson WD, Linnarsson S, Castelo-Branco G (2016) Oligodendrocyte heterogeneity in the mouse juvenile and adult central nervous system. Science 352(6291):1326–1329. https://doi.org/10.1126/science.aaf6463
Marton RM, Miura Y, Sloan SA, Li Q, Revah O, Levy RJ et al (2019) Differentiation and maturation of oligodendrocytes in human three-dimensional neural cultures. Nat Neurosci 22(3):484–491. https://doi.org/10.1038/s41593-018-0316-9
Marx U, Akabane T, Andersson TB, Baker E, Beilmann M, Beken S et al (2020) Biology-inspired microphysiological systems to advance patient benefit and animal welfare in drug development. ALTEX 37(3):364–394. https://doi.org/10.14573/altex.2001241
Marx U, Andersson TB, Bahinski A, Beilmann M, Beken S, Cassee FR et al (2016) Biology-inspired microphysiological system approaches to solve the prediction dilemma of substance testing. ALTEX 33(3):272–321. https://doi.org/10.14573/altex.1603161
Milligan CJ, Li J, Sukumar P, Majeed Y, Dallas ML, English A, Emery P, Porter KE, Smith AM, McFadzean I, Beccano-Kelly D, Bahnasi Y, Cheong A, Naylor J, Zeng F, Liu X, Gamper N, Jiang LH, Pearson HA, Peers C, Robertson B, Beech DJ (2009) Robotic multiwell planar patch-clamp for native and primary mammalian cells. Nat Protoc 4(2):244–255. https://doi.org/10.1038/nprot.2008.230
Mitani A, Komiyama T (2018) Real-time processing of two-photon calcium imaging data including lateral motion artifact correction. Front Neuroinform 12:98. https://doi.org/10.3389/fninf.2018.00098
Mohs RC, Greig NH (2017) Drug discovery and development: role of basic biological research. Alzheimers Dement (N Y) 3(4):651–657. https://doi.org/10.1016/j.trci.2017.10.005
Monje PV (2020) Schwann cell cultures: biology, technology and therapeutics. Cells 9(8). https://doi.org/10.3390/cells9081848
Morante-Redolat JM, Porlan E (2019) Neural stem cell regulation by adhesion molecules within the subependymal niche. Front Cell Dev Biol 7:102. https://doi.org/10.3389/fcell.2019.00102
Moutaux E, Charlot B, Genoux A, Saudou F, Cazorla M (2018) An integrated microfluidic/microelectrode array for the study of activity-dependent intracellular dynamics in neuronal networks. Lab Chip 18(22):3425–3435. https://doi.org/10.1039/c8lc00694f
Musto M, Rauti R, Rodrigues AF, Bonechi E, Ballerini C, Kostarelos K, Ballerini L (2019) 3D organotypic spinal cultures: exploring neuron and neuroglia responses upon prolonged exposure to graphene oxide. Front Syst Neurosci 13:1. https://doi.org/10.3389/fnsys.2019.00001
Najjar S, Pearlman DM (2015) Neuroinflammation and white matter pathology in schizophrenia: systematic review. Schizophr Res 161(1):102–112. https://doi.org/10.1016/j.schres.2014.04.041
Nayernia Z, Turchi L, Cosset E, Peterson H, Dutoit V, Dietrich PY, Tirefort D, Chneiweiss H, Lobrinus JA, Krause KH, Virolle T, Preynat-Seauve O (2013) The relationship between brain tumor cell invasion of engineered neural tissues and in vivo features of glioblastoma. Biomaterials 34(33):8279–8290. https://doi.org/10.1016/j.biomaterials.2013.07.006
Nguyen H, McCoy L, Willey H, Sharma AD, Curley L, Moore M (2019) Nerve-on-a-chip platform for assessing chemotherapy-induced peripheral neuropathy. J Pharmacol Toxicol Methods 99:106595. https://doi.org/10.1016/j.vascn.2019.05.088
Nikolakopoulou P, Rauti R, Voulgaris D, Shlomy I, Maoz BM, Herland A (2020) Recent progress in translational engineered in vitro models of the central nervous system. Brain. https://doi.org/10.1093/brain/awaa268
Nirwane A, Yao Y (2018) Laminins and their receptors in the CNS. Biol Rev Camb Philos Soc 94:283–306. https://doi.org/10.1111/brv.12454
Noseworthy JH, Lucchinetti C, Rodriguez M, Weinshenker BG (2000) Multiple sclerosis. N Engl J Med 343(13):938–952. https://doi.org/10.1056/NEJM200009283431307
Nzou G, Wicks RT, Wicks EE, Seale SA, Sane CH, Chen A, Murphy SV, Jackson JD, Atala AJ (2018) Human cortex spheroid with a functional blood brain barrier for high-throughput neurotoxicity screening and disease modeling. Sci Rep 8(1):7413. https://doi.org/10.1038/s41598-018-25603-5
Obien ME, Deligkaris K, Bullmann T, Bakkum DJ, Frey U (2014) Revealing neuronal function through microelectrode array recordings. Front Neurosci 8:423. https://doi.org/10.3389/fnins.2014.00423
Ogawa J, Pao GM, Shokhirev MN, Verma IM (2018) Glioblastoma model using human cerebral organoids. Cell Rep 23(4):1220–1229. https://doi.org/10.1016/j.celrep.2018.03.105
Omar A, Marwaha K, Bollu PC (2020) Physiology, neuromuscular junction. In StatPearls. Treasure Island (FL)
Osaki T, Uzel SGM, Kamm RD (2020) On-chip 3D neuromuscular model for drug screening and precision medicine in neuromuscular disease. Nat Protoc 15(2):421–449. https://doi.org/10.1038/s41596-019-0248-1
Pamies D, Barreras P, Block K, Makri G, Kumar A, Wiersma D, … Hogberg HT (2017). A human brain microphysiological system derived from induced pluripotent stem cells to study neurological diseases and toxicity. ALTEX 34(3):362–376. https://doi.org/10.14573/altex.1609122
Pamies D, Block K, Lau P, Gribaldo L, Pardo CA, Barreras P, Smirnova L, Wiersma D, Zhao L, Harris G, Hartung T, Hogberg HT (2018) Rotenone exerts developmental neurotoxicity in a human brain spheroid model. Toxicol Appl Pharmacol 354:101–114. https://doi.org/10.1016/j.taap.2018.02.003
Pellegrini L, Bonfio C, Chadwick J, Begum F, Skehel M, Lancaster MA (2020) Human CNS barrier-forming organoids with cerebrospinal fluid production. Science. 369:eaaz5626. https://doi.org/10.1126/science.aaz5626
Pistollato, F., Ohayon, E. L., Lam, A., Langley, G. R., Novak, T. J., Pamies, D., … Chandrasekera, P. C. (2016). Alzheimer disease research in the 21st century: past and current failures, new perspectives and funding priorities. Oncotarget, 7(26), 38999-39016. doi:https://doi.org/10.18632/oncotarget.9175
Plummer S, Wallace S, Ball G, Lloyd R, Schiapparelli P, Quinones-Hinojosa A et al (2019) A Human iPSC-derived 3D platform using primary brain cancer cells to study drug development and personalized medicine. Sci Rep 9(1):1407. https://doi.org/10.1038/s41598-018-38130-0
Pollard K, Sharma AD, Moore M (2019) Neural microphysiological systems for in vitro modeling of peripheral nervous system disorders. Bioelectron Med 2:101–117. https://doi.org/10.2217/bem-2019-0018
Previtera ML, Langhammer CG, Firestein BL (2010) Effects of substrate stiffness and cell density on primary hippocampal cultures. J Biosci Bioeng 110(4):459–470. https://doi.org/10.1016/j.jbiosc.2010.04.004
Qian X, Nguyen HN, Song MM, Hadiono C, Ogden SC, Hammack C, Yao B, Hamersky GR, Jacob F, Zhong C, Yoon KJ, Jeang W, Lin L, Li Y, Thakor J, Berg DA, Zhang C, Kang E, Chickering M, Nauen D, Ho CY, Wen Z, Christian KM, Shi PY, Maher BJ, Wu H, Jin P, Tang H, Song H, Ming GL (2016) Brain-region-specific organoids using mini-bioreactors for modeling ZIKV exposure. Cell 165(5):1238–1254. https://doi.org/10.1016/j.cell.2016.04.032
Renault R, Sukenik N, Descroix S, Malaquin L, Viovy JL, Peyrin JM, Bottani S, Monceau P, Moses E, Vignes M (2015) Combining microfluidics, optogenetics and calcium imaging to study neuronal communication in vitro. PLoS One 10(4):e0120680. https://doi.org/10.1371/journal.pone.0120680
Rouwkema J, Koopman B, Blitterswijk C, Dhert W, Malda J (2010) Supply of nutrients to cells in engineered tissues. Biotechnol Genet Eng Rev 26:163–178. https://doi.org/10.5661/bger-26-163
Sakai K, Shimba K, Kotani K, Jimbo Y (2017) A co-culture microtunnel technique demonstrating a significant contribution of unmyelinated Schwann cells to the acceleration of axonal conduction in Schwann cell-regulated peripheral nerve development. Integr Biol (Camb) 9(8):678–686. https://doi.org/10.1039/c7ib00051k
Santhanam N, Kumanchik L, Guo X, Sommerhage F, Cai Y, Jackson M, Martin C, Saad G, McAleer CW, Wang Y, Lavado A, Long CJ, Hickman JJ (2018) Stem cell derived phenotypic human neuromuscular junction model for dose response evaluation of therapeutics. Biomaterials 166:64–78. https://doi.org/10.1016/j.biomaterials.2018.02.047
Savtchouk I, Carriero G, Volterra A (2018) Studying axon-astrocyte functional interactions by 3D two-photon Ca(2+) imaging: a practical guide to experiments and “big data” analysis. Front Cell Neurosci 12:98. https://doi.org/10.3389/fncel.2018.00098
Schafer DP, Lehrman EK, Stevens B (2013) The “quad-partite” synapse: microglia-synapse interactions in the developing and mature CNS. Glia 61(1):24–36. https://doi.org/10.1002/glia.22389
Schmidt BZ, Lehmann M, Gutbier S, Nembo E, Noel S, Smirnova L, … Dinnyes A (2017) In vitro acute and developmental neurotoxicity screening: an overview of cellular platforms and high-throughput technical possibilities. Arch Toxicol, 91(1):1-33. doi:https://doi.org/10.1007/s00204-016-1805-9
Sharma AD, McCoy L, Jacobs E, Willey H, Behn JQ, Nguyen H, Bolon B, Curley JL, Moore MJ (2019) Engineering a 3D functional human peripheral nerve in vitro using the nerve-on-a-chip platform. Sci Rep 9(1):8921. https://doi.org/10.1038/s41598-019-45407-5
Shin Y, Choi SH, Kim E, Bylykbashi E, Kim JA, Chung S, Kim DY, Kamm RD, Tanzi RE (2019) Blood-brain barrier dysfunction in a 3D in vitro model of Alzheimer’s disease. Adv Sci (Weinh) 6(20):1900962. https://doi.org/10.1002/advs.201900962
Simao D, Terrasso AP, Teixeira AP, Brito C, Sonnewald U, Alves PM (2016) Functional metabolic interactions of human neuron-astrocyte 3D in vitro networks. Sci Rep 6:33285. https://doi.org/10.1038/srep33285
Sloan SA, Darmanis S, Huber N, Khan TA, Birey F, Caneda C, … Pasca SP (2017) Human astrocyte maturation captured in 3D cerebral cortical spheroids derived from pluripotent stem cells. Neuron, 95(4):779-790 e776. doi:https://doi.org/10.1016/j.neuron.2017.07.035
Smirnova L, Hogberg HT, Leist M, Hartung T (2014) Developmental neurotoxicity - challenges in the 21st century and in vitro opportunities. ALTEX 31(2):129–156. https://doi.org/10.14573/altex.1403271
Soscia DA, Lam D, Tooker AC, Enright HA, Triplett M, Karande P, Peters SKG, Sales AP, Wheeler EK, Fischer NO (2020) A flexible 3-dimensional microelectrode array for in vitro brain models. Lab Chip 20(5):901–911. https://doi.org/10.1039/c9lc01148j
Tanaka A, Fujii Y, Kasai N, Okajima T, Nakashima H (2018) Regulation of neuritogenesis in hippocampal neurons using stiffness of extracellular microenvironment. PLoS One 13(2):e0191928. https://doi.org/10.1371/journal.pone.0191928
Tremblay ME, Cookson MR, Civiero L (2019) Glial phagocytic clearance in Parkinson’s disease. Mol Neurodegener 14(1):16. https://doi.org/10.1186/s13024-019-0314-8
Trujillo CA, Gao R, Negraes PD, Gu J, Buchanan J, Preissl S, Wang A, Wu W, Haddad GG, Chaim IA, Domissy A, Vandenberghe M, Devor A, Yeo GW, Voytek B, Muotri AR (2019) Complex oscillatory waves emerging from cortical organoids model early human brain network development. Cell Stem Cell 25(4):558–569 e557. https://doi.org/10.1016/j.stem.2019.08.002
Tuft BW, Zhang L, Xu L, Hangartner A, Leigh B, Hansen MR, Guymon CA (2014) Material stiffness effects on neurite alignment to photopolymerized micropatterns. Biomacromolecules 15(10):3717–3727. https://doi.org/10.1021/bm501019s
Ulloa Severino FP, Ban J, Song Q, Tang M, Bianconi G, Cheng G, Torre V (2016) The role of dimensionality in neuronal network dynamics. Sci Rep 6:29640. https://doi.org/10.1038/srep29640
Vereyken EJ, Fluitsma DM, Bolijn MJ, Dijkstra CD, Teunissen CE (2009) An in vitro model for de- and remyelination using lysophosphatidyl choline in rodent whole brain spheroid cultures. Glia 57(12):1326–1340. https://doi.org/10.1002/glia.20852
Villabona-Rueda A, Erice C, Pardo CA, Stins MF (2019) The evolving concept of the blood brain barrier (BBB): from a single static barrier to a heterogeneous and dynamic relay center. Front Cell Neurosci 13:405. https://doi.org/10.3389/fncel.2019.00405
Walsh P, Truong V, Hill C, Stoflet ND, Baden J, Low WC, Keirstead SA, Dutton JR, Parr AM (2017) Defined culture conditions accelerate small-molecule-assisted neural induction for the production of neural progenitors from human-induced pluripotent stem cells. Cell Transplant 26(12):1890–1902. https://doi.org/10.1177/0963689717737074
Wen YQ, Gao X, Wang A, Yang Y, Liu S, Yu Z, Song GB, Zhao HC (2018) Substrate stiffness affects neural network activity in an extracellular matrix proteins dependent manner. Colloids Surf B: Biointerfaces 170:729–735. https://doi.org/10.1016/j.colsurfb.2018.03.042
Xiang Y, Tanaka Y, Patterson B, Kang YJ, Govindaiah G, Roselaar N, Cakir B, Kim KY, Lombroso AP, Hwang SM, Zhong M, Stanley EG, Elefanty AG, Naegele JR, Lee SH, Weissman SM, Park IH (2017) Fusion of regionally specified hPSC-derived organoids models human brain development and interneuron migration. Cell Stem Cell 21(3):383–398 e387. https://doi.org/10.1016/j.stem.2017.07.007
Xu H, Li Z, Yu Y, Sizdahkhani S, Ho WS, Yin F, Wang L, Zhu G, Zhang M, Jiang L, Zhuang Z, Qin J (2016) A dynamic in vivo-like organotypic blood-brain barrier model to probe metastatic brain tumors. Sci Rep 6:36670. https://doi.org/10.1038/srep36670
Yang W, Miller JE, Carrillo-Reid L, Pnevmatikakis E, Paninski L, Yuste R, Peterka DS (2016) Simultaneous multi-plane imaging of neural circuits. Neuron 89(2):269–284. https://doi.org/10.1016/j.neuron.2015.12.012
Zhan J, Mann T, Joost S, Behrangi N, Frank M, Kipp M (2020) The cuprizone model: dos and do nots. Cells 9(4). https://doi.org/10.3390/cells9040843
Zhang QY, Zhang YY, Xie J, Li CX, Chen WY, Liu BL, Wu XA, Li SN, Huo B, Jiang LH, Zhao HC (2014) Stiff substrates enhance cultured neuronal network activity. Sci Rep 4:6215. https://doi.org/10.1038/srep06215
Zhong X, Harris G, Smirnova L, Zufferey V, Sa R, Baldino Russo F et al (2020) Antidepressant paroxetine exerts developmental neurotoxicity in an iPSC-derived 3D human brain model. Front Cell Neurosci 14:25. https://doi.org/10.3389/fncel.2020.00025
Zonouzi M, Berger D, Jokhi V, Kedaigle A, Lichtman J, Arlotta P (2019) Individual oligodendrocytes show bias for inhibitory axons in the neocortex. Cell Rep 27(10):2799–2808 e2793. https://doi.org/10.1016/j.celrep.2019.05.018
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
WAA is a paid employee of AxoSim, Inc., New Orleans, LA, USA, which is a for-profit entity commercializing neural microphysiological system. TH and HH are inventors on a patent by Johns Hopkins University, which is licensed to AxoSim, for which they both consult and TH is a shareholder. MJM is an inventor on a patent by Tulane University also licensed by AxoSim, of which he is a co-founder and shareholder. AB declares no conflict of interest.
Additional information
Editor: Tetsuji Okamoto
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Anderson, W.A., Bosak, A., Hogberg, H.T. et al. Advances in 3D neuronal microphysiological systems: towards a functional nervous system on a chip. In Vitro Cell.Dev.Biol.-Animal 57, 191–206 (2021). https://doi.org/10.1007/s11626-020-00532-8
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11626-020-00532-8