Abstract
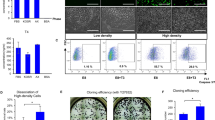
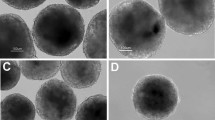
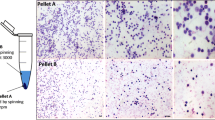
Leptin, a metabolic hormone, regulates the reproductive functions responding to both nutritional and body conditions. Embryonic stem cells play important roles in reproductive technology, but their derivation can be challenging. In this study, we evaluated the derivation rates of mouse embryonic stem cell (mESC) line from blastocysts developing in embryo culture media supplemented with different leptin concentrations. The results showed that addition of leptin into the embryo culture medium supported the in vitro development of mouse embryo. The mESC line derivation rates for media treated with 0, 10, 50, and 100 ng/ml of leptin were 61.24 % (54/88), 84.96 % (42/50), 81.79 % (61/76), and 85.78 % (56/67), respectively. In addition, leptin treatment of blastocysts upregulated the expression levels of the trophectoderm marker Cdx2, whereas inner cell mass markers Oct-4 and Nanog were not affected. mESC lines derived after leptin treatment demonstrated hallmarks of pluripotency, such as alkaline phosphatase activity, expression of, OCT4, NANOG, and SSEA1, as well as the ability to form embryoid bodies and well-differentiated teratomas. In conclusion, leptin has a positive effect on the derivation rate of mouse embryonic stem cell lines which may be, in part, due to its effects on the development of the trophectoderm cell lineage in the embryo.



Similar content being viewed by others
References
Bagis H, Akkoc T, Taskin C, Arat S (2010) Comparison of different cryopreservation techniques: higher survival and implantation rate of frozen–thawed mouse pronuclear embryos in the presence of beta-mercaptoethanol in post-thaw culture. Reprod Domest Anim 45:332–337
Cobler L, Pera M, Garrido M, Iglesias M, de Bolós C (2014) CDX2 can be regulated through the signalling pathways activated by IL-6 in gastric cells. Biochim Biophys Acta 1839:785–792
Coskun M, Olsen AK, Bzorek M, Holck S, Engel UH, Nielsen OH, Troelsen JT (2014) Involvement of CDX2 in the cross talk between TNF-α and Wnt signaling pathway in the colon cancer cell line Caco-2. Carcinogenesis 35:1185–1192
Costa-Borges N, Santaló J, Ibáñez E (2010) Comparison between the effect of valproic acid and trichostatin A on the in vitro development, blastocyst quality, and full-term development of mouse somatic cell nuclear transfer embryos. Cell Reprogram 12(4):437–46
Czechanski A, Byers C, Greenstein I, Schrode N, Donahue LR, Hadjantonakis AK, Reinholdt L (2014) Derivation and characterization of mouse embryonic stem cells from permissive and nonpermissive strains. Nat Protoc 9:559–574
Evans MJ, Kaufman MF (1981) Establishment in culture of pluripotential cells from mouse embryos. Nature 292:154–156
Fedorcsák P, Dale PO, Storeng R, Ertzeid G, Bjercke S, Oldereid N, Omland AK, Åbyholm T, Tanbo T (2004) Impact of overweight and underweight on assisted reproduction treatment. Hum Reprod 19:2523–2528
Feldman DE, Chen C, Punj V, Tsukamoto H, Machida K (2012) Pluripotency factor-mediated expression of the leptin receptor (OB-R) links obesity to oncogenesis through tumor-initiating stem cells. Proc Natl Acad Sci U S A 109:829–834
Fidan K, Kavaklıoğlu G, Ebrahimi A, Özlü C, Ay NZ, Ruacan A, Gül A, Önder TT (2015) Generation of integration-free induced pluripotent stem cells from a patient with Familial Mediterranean Fever (FMF). Stem Cell Res 15:694–696
Herrid M, Nguyen VL, Hinch G, McFarlane JR (2006) Leptin has concentration and stage-dependent effects on embryonic development in vitro. Reproduction 132:247–256
Iijima S, Tanimoto Y, Mizuno S, Daitoku Y, Kunita S, Sugiyama F, Yagami KI (2010) Effect of different culture conditions on establishment of embryonic stem cells from BALB/cAJ and NZB/BINJ mice. Cell Rep 12:679–688
Inagaki-Ohara K, Okamoto S, Takagi K, Saito K, Arita S, Tang L, Hori T, Kataoka H, Matsumoto S, Minokoshi Y (2016) Leptin receptor signaling is required for high-fat diet-induced atrophic gastritis in mice. Nutr Metab 13:7
Kanda A, Sotomaru Y, Shiozawa S, Hiyama E (2012) Establishment of ES cells from inbred strain mice by dual inhibition (2i). J Reprod Dev 58:77–83
Kawamura K, Sato N, Fukuda J, Kodama H, Kumagai J, Tanikawa H, Murata M, Tanaka T (2003) The role of leptin during the development of mouse preimplantation embryos. Mol Cell Endocrinol 202:185–189
Kawamura K, Sato N, Fukuda J, Kodama H, Kumagai J, Tanikawa H, Nakamura A, Tanaka T (2002) Leptin promotes the development of mouse preimplantation embryos in vitro. Endocrinology 143:1922–1931
Liu J, Schoonjans L, Tielens S, Speleman F, Cornelissen M, De Sutter P, Dhont MARC, Van der Elst J (2006) Culturing in vitro produced blastocysts in sequential media promotes ES cell derivation. Mol Reprod Dev 73:1017–1021
Livak KJ, Schmittgen TD (2001) Analysis of relative gene expression data using real-time quantitative PCR and the 2− ΔΔCT method. Methods 25:402–408
Lu CW, Yabuuchi A, Chen L, Viswanathan S, Kim K, Daley GQ (2008) Ras-MAPK signaling promotes trophectoderm formation from embryonic stem cells and mouse embryos. Nat Genet 40(7):921–926
Ma Z, Swigut T, Valouev A, Rada-Iglesias A, Wysocka J (2011) Sequence-specific regulator Prdm14 safeguards mouse ESCs from entering extraembryonic endoderm fates. Nat Struct Mol Biol 18:120–127
Mallol A, Santaló J, Ibáñez E (2013) Comparison of three differential mouse blastocyst staining methods. Syst Biol Reprod Med 59:117–122
Martin GR (1981) Isolation of a pluripotent cell line from early mouse embryos cultured in medium conditioned by teratocarcinoma stem cells. Proc Natl Acad Sci US A 78:7634–7638
Nichols J, Chambers I, Smith A (1994) Derivation of germline competent embryonic stem cells with a combination of interleukin-6 and soluble interleukin-6 receptor. Exp Cell Res 215:237–239
Nichols J, Evans EP, Smith AG (1990) Establishment of germ-line-competent embryonic stem (ES) cells using differentiation inhibiting activity. Development 110:1341–1348
Park HK, Ahima RS (2014) Leptin signaling. F1000 Prime Rep 6:73
Robertson EJ (1987) Embryo-derived stem cell lines. Teratocarcinomas and embryonic stem cells a practical approach 71–112
Rungsiwiwut R, Rungarunlert S, Numchaisrika P, Pruksananonda K, Techakumphu M, Virutamasen P (2008) Effect of leukemia inhibitory factor (LIF) on the quality of in vitro produced mouse blastocysts and subsequent derivation of embryonic stem (ES) cells. J Med Assoc Thai 91:608–614
Smith AG (2001) Embryo-derived stem cells: of mice and men. Annu Rev Cell Dev Biol 17:435–462
Taskin AC, Akkoc T, Sagirkaya H, Bagis H, Arat S (2016) Comparison of the development of mouse embryos manipulated with different biopsy techniques. Turk J Vet Anim Sci 40:157–162
Thouas GA, Korfiatis NA, French AJ, Jones GM, Trounson AO (2001) Simplified technique for differential staining of inner cell mass and trophectoderm cells of mouse and bovine blastocysts. Reprod BioMed Online 3:25–29
Tielens S, Verhasselt B, Liu J, Dhont MARC, Van der Elst J, Cornelissen M (2006) Generation of embryonic stem cell lines from mouse blastocysts developed in vivo and in vitro: relation to Oct-4 expression. Reproduction 132:59–66
Wu G, Gentile L, Do JT, Cantz T, Sutter J, Psathaki K, Araúzo-Bravo MJ, Ortmeier C, Schöler HR (2010a) Efficient derivation of pluripotent stem cells from siRNA-mediated Cdx2-deficient mouse embryos. Stem Cells Dev 20(3):485–493
Wu G, Gentile L, Fuchikami T, Sutter J, Psathaki K, Esteves TS, Araúzo-Bravo MJ, Ortmeier C, Verberk G, Abe K, Schöler HR (2010b) Initiation of trophectoderm lineage specification in mouse embryos is independent of Cdx2. Development 134:4159–4169
Yang YJ, Cao YJ, Bo SM, Peng S, Liu WM, Duan EK (2006) Leptin-directed embryo implantation: leptin regulates adhesion and outgrowth of mouse blastocysts and receptivity of endometrial epithelial cells. Anim Reprod Sci 92(1–2):155–167
Zhang Y, Proenca R, Maffei M, Barone M, Leopold L, Friedman JM (1994) Positional cloning of the mouse obese gene and its human homologue. Nature 372:425–432
Acknowledgments
The authors gratefully acknowledge use of the services and facilities of the Koç University Research Center for Translational Medicine (KUTTAM), funded by the Republic of Turkey Ministry of Development. The content is solely the responsibility of the authors and does not necessarily represent the official views of the Ministry of Development.
Funding
This research supported by a grant from TUBITAK - The Scientific and Technological Research Council of Turkey (Grant Number: TOVAG 113O223).
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
All mouse experiments and animal care protocols were approved by the Local Ethics Committee for Animal Experiments (Approval No: 2013-06), Koç University.
Conflict of interest
The authors declare that they have no competing interests.
Additional information
Editor: Tetsuji Okamoto
Prior conference presentation of the submitted: Taskin AC, A Kocabay, TT Onder and A Ebrahimi. (2016) Effect of leptin on derivation rate of mouse embryonic stem (ES) cell line. In TRANSGENIC RESEARCH M 25: 265–265 (13th Transgenic Technology Meeting in Prague, Czech Republic, from March 20th–23th, 2016)
Rights and permissions
About this article
Cite this article
Taskin, A.C., Kocabay, A., Ebrahimi, A. et al. Leptin treatment of in vitro cultured embryos increases outgrowth rate of inner cell mass during embryonic stem cell derivation. In Vitro Cell.Dev.Biol.-Animal 55, 473–481 (2019). https://doi.org/10.1007/s11626-019-00367-y
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11626-019-00367-y