Abstract
Background
In 2012, the US Preventive Services Task Force (USPSTF) recommended against PSA-based screening for prostate cancer in men of all ages. Following this change, screening declined yet the complete impact on clinical presentation is not well defined in the screen-eligible population.
Objective
To determine if the rates of PSA screening, prostate biopsy, incident prostate cancer detection, and stage IV at presentation in screen-eligible men in Kaiser Permanente Northern California changed following the 2012 USPSTF Prostate Cancer Screening recommendations.
Design
Retrospective study spanning the years 2010 to 2015, in screen-eligible Kaiser Permanente Northern California members (African American men ages 45–69 and all other men ages 50–69) with no prior history of prostate cancer.
Participants
All screen-eligible, male members during 2010 (n = 403,931) to 2015 (n = 483,286) without a history of prostate cancer within all Kaiser Permanente Northern California facilities.
Main Measures
Annual rates of PSA testing, prostate biopsy, incident prostate cancer detection, and stage IV cancer at presentation were compared between the pre-guideline period, 2010 and 2011, and the post-guideline period, 2014 and 2015, in men under the age of 70.
Key Results
Following the 2012 USPSTF guideline change, screening rates declined 23.4% (95% CI 23.0–23.8%), biopsy rates declined 64.3% (95% CI 62.9–65.6%), and incident prostate cancer detection rates declined 53.5% (95% CI 50.1–56.7%) resulting in 1871 fewer incident cancers detected, and metastatic cancer rates increased 36.9% (95% CI 9.5–71.0%) resulting in 75 more stage IV cancers detected.
Conclusion
Less screening resulted in a large decrease in cancer detection, some of which may be beneficial as many cancers may be indolent, yet this decrease occurred at the expense of an increase in metastatic cancer rates. For every 25 fewer cancers detected, one metastatic cancer was diagnosed. This information may be valuable in the shared decision-making process around prostate cancer screening.
Similar content being viewed by others
Explore related subjects
Discover the latest articles and news from researchers in related subjects, suggested using machine learning.Avoid common mistakes on your manuscript.
INTRODUCTION
Prostate cancer is the most common cancer in American men and the second leading cause of cancer death [1]. The heterogeneity of disease biology spanning from indolent to aggressive, coupled with more intensive detection strategies can result in over-detection which, if linked to over-treatment, results in unnecessary interventions. These interventions, including both surgery and radiation therapy, can have a negative impact upon bowel, bladder and/or sexual function. Disparate results from large screening trials have led to questioning the value of screening [2, 3]. In May 2012, the US Preventive Services Task Force (USPSTF) gave a “D” grade (“Discourage the use of- this service”) to PSA-based screening for prostate cancer in all age groups [4]. While many organizations provide screening guidelines, primary care physicians tend to favor USPSTF guidelines [5]. Recently, the USPSTF upgraded prostate cancer screening for men ages 55–69 to a Grade “C” (“No recommendation for or against routine provision of this service”) [6].
Many studies have demonstrated a decline in prostate cancer screening following release of the 2012 statement with absolute annual screening rates ranging from 23–45% pre-guideline to 17–35% post-guideline, and relative reductions ranging from 15 to 28% [7,8,9,10,11]. Studies have also demonstrated relative reductions in prostate biopsy ranging from 22 to 38% [12,13,14,15,16]. Limitations of these studies include use of self-reported data, single-group practices, administrative claims, or a lack of information on health-plan enrollment, and thus an inability to calculate a denominator needed for accurate rates.
Changes in screening and biopsy rates influence prostate cancer detection. Analyses using the Surveillance, Epidemiology and End Results (SEER) database have shown a 16% relative decline in the incidence of non-metastatic prostate cancer from 2011 to 2012 and an additional 6% relative decline from 2012 to 2013 [7, 17]. Data regarding metastatic rates is contradictory. Two registry studies using the Pennsylvania Cancer Registry and SEER data did not show an increase in metastatic cancer rates although their follow-up spanned only the period from 2012 to 2013 [17, 18]. Another study using SEER data, focusing on men 75 years of age and older, showed an increase in metastatic disease [19]. A recent study using SEER registries and the North American Association of Central Cancer Registries did show an increase in advanced stages of the disease in men ages 50–74 and in men 75 years of age and older [20]. Another SEER study found an increase in metastatic rates from 2012 to 2014 in men aged 55–69 and used age-period-cohort models to estimate future rates, but the authors acknowledged that they did not have any PSA-screening data to determine whether a decline in screening was likely to be causal [21]. At present, most guidelines recommend against screening men 70 years of age and older, thus it is critical to understand the impact of any screening policy changes in men under the age of 70. Data on rates of metastatic disease as it relates to the intensity of screening in American men under the age of 70 is lacking.
Our study sought to quantify rates of PSA testing, biopsy, and incident prostate cancer and stage IV cancer before and after the 2012 USPSTF recommendation change by examining a large, representative population of screen-eligible men within a large integrated health care system. Since most primary care providers tend to follow USPSTF guidelines in preference to any other internal or external guidelines, the 2012 USPSTF Statement provided an opportunity to observe a “natural experiment” to observe how it affected screening, biopsy cancer detection and metastatic rates. Specifically, we analyzed only “screen-eligible” men. These results provide critical elements for the shared decision-making process currently recommended by most organizations.
METHODS
Study Population
We performed a retrospective study of KPNC electronic medical records encompassing the years 2010 to 2015. KPNC is a large integrated health care system that provides comprehensive medical services to over 4 million members. The study population was all screen-eligible KPNC members defined as African American men aged 45–69 and all other men aged 50–69. Men with a previous diagnosis of prostate cancer were excluded. Members were required to have a minimum of 3 months of health-plan enrollment in a given year in order to have an opportunity to be offered screening in that year. Variables that could affect rates of cancer and metastatic cancer including age and race and those that could affect screening including comorbidities (COmorbidity Patient Score Version 2 [COPS2]) [22], smoking status, body mass index (BMI), and utilization of care (number of primary care visits per year) were measured.
Screening Guidelines at KPNC
KPNC has its own National Prostate Cancer Screening Clinical Guideline which did not substantially change between 2010 and 2015 and that advocates shared decision-making. This guideline states that in average-risk men with at least a 10-year life expectancy, offering PSA-based prostate cancer screening is an option for men ages 50–69. For higher-risk men (Black/African American descent or family history of prostate cancer), screening is an option for ages 45–69. The KPNC guideline, while available to providers, is not monitored or enforced by any means and providers practice independently.
Outcomes
Four outcomes were studied: PSA screening, prostate biopsy, incident prostate cancer, and incident metastatic prostate cancer (American Joint Committee on Cancer Stage IV) [23]. PSA screening and prostate biopsy information was obtained from patient electronic medical records. Prostate cancer cases were ascertained from the KPNC Regional Cancer Registry. This registry is accredited by SEER, the California Cancer Registry and the Commission on Cancer.
Exposure
The main exposure of interest for this study was the 2012 USPSTF Prostate Cancer Screening recommendations. This guideline was released as a draft statement in October of 2011 and finalized in May of 2012. Because adoption of guidelines often occurs slowly over time, we a priori identified three time periods: the pre-guideline period included years 2010 and 2011; the transition period included years 2012 and 2013; and the post-guideline period included years 2014 and 2015. All rate comparisons were between the pre-guideline and post-guideline periods.
Statistical Analysis
We computed annual rates of PSA testing and prostate biopsy. All screen-eligible men within a given year were included in the denominator in all rate calculations accounting for the duration of enrollment within that year (person-years at risk). Rate ratios (RR) and 95% confidence intervals (CIs) were calculated to compare rates of PSA testing and prostate biopsy between the two time periods. For incident prostate cancer and metastatic disease, we computed crude and adjusted annual incidence rates using a Poisson model accounting for overdispersion. Incidence rate ratios (IRR) and 95% CIs were calculated to compare rates of incident prostate cancer and incident stage IV prostate cancer between the two time periods. We conducted a sensitivity analysis of incident prostate cancer and stage IV cancer rates by restricting our analyses to men who were enrolled in our health plan for a minimum of 12 months. We conducted an interrupted time series analysis (ITS) to evaluate the effect of introducing the 2012 USPSTF guideline as a population-level intervention on incident prostate cancer and incident stage IV prostate cancer [24]. Two-sided p values < 0.05 were considered statistically significant. Analyses were conducted in SAS 9.3 and R 3.4.4.
RESULTS
Table 1 shows the demographics of screen-eligible KPNC members in the pre-guideline period and the post-guideline period. We looked at variables that might influence rates of cancer or metastatic cancer as well as other factors that might influence screening and care utilization. The demographics were very similar over the study time period. p values are not reported in Table 1 because even extremely small differences in the population characteristics that are not clinically significant would be statistically significant under this large sample size [25].
Table 2 shows annual PSA screening and biopsy rates among screen-eligible men, along with corresponding PSA levels. The median PSA levels of men undergoing screening appeared to be stable over time. There was a statistically significant decline in screening rates from the pre-guideline to the post-guideline period with an RR = 0.766, (95% CI 0.762 to 0.770). There was a large and statistically significant decline in biopsy rates from the pre-guideline to the post-guideline period with an RR of 0.357 (95% CI 0.344 to 0.371).
Table 3 shows the annual number of incident and stage IV incident prostate cancers detected, the associated incidence rates, and corresponding PSA levels. From 2010 through 2015, there were 9741 new cases of prostate cancer diagnosed of which 448 were stage IV. There was a large statistically significant decline in incident prostate cancer detection rates from the pre-guideline to the post-guideline period with an unadjusted IRR of 0.467 (95% CI 0.443 to 0.492) and an age and race-adjusted IRR of 0.465 (95% CI 0.433 to 0.499). There was a large statistically significant increase in incident stage IV cancer detection rates from the pre-guideline period to the post-guideline period with an unadjusted IRR of 1.387 (95% CI 1.110 to 1.733) and an age and race-adjusted IRR of 1.369 (95% CI 1.095 to 1.710). Of the 448 men with incident metastatic prostate cancer over the study period, most men had stable KPNC membership before diagnosis: 108 (24.1%) were members for > 5 years, 173 men (38.6%) were members for 2–5 years, 63 men (14.1%) were members for 1–2 years and 104 men (23.2%) had membership of less than 12 months.
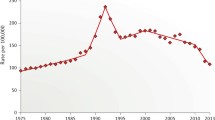
Figure 1 shows the results of the interrupted time series analysis. Incident prostate cancer rates showed a decreasing trend during the pre-guideline (2010–2011) and transition (2012–2013) periods. During the post-guideline period, there was a substantially lower new constant rate; this change in trend between the pre- and transition periods to the post-guideline period was statistically significant (p < 0.0001). Incident metastatic disease rates were constant during the pre-guideline (2010–2011) and transition (2012–2013) periods. This rate changed in the post-guideline period to a new constant rate, and the difference in the two rates was statistically significant (p < 0.0001).
Interrupted time series analysis. Incident prostate cancer rates (left panel) declined in the pre-guideline and “transition” period and reached a lower, constant rate in the “post-guideline” period. Incident metastatic rates (right panel) were stable in pre-guideline and “transition” period and reached a higher, constant rate in the “post-guideline” period.
We conducted a sensitivity analysis including only men enrolled in the health plan for at least 12 months prior to diagnosis (to allow sufficient opportunity for screening to be offered, Table 4). This analysis revealed similar trends in cancer rates to those observed for the entire screen-eligible population. There was a statistically significant decline in incident prostate cancer detection rates from the pre-guideline to the post-guideline period with an age and race-adjusted IRR of 0.470 (95% CI 0.437 to 0.506). There was a statistically significant increase in stage IV cancer detection rates from the pre-guideline to the post-guideline period with an age and race-adjusted IRR of 1.531 (95% CI 1.182 to 1.982).
DISCUSSION
In a study of electronic medical records in men under the age of 70 from a large healthcare system, we found that following the 2012 USPSTF guideline change, relative screening rates for prostate cancer declined 23.4%, prostate biopsy rates declined 64.3%, incident prostate cancer detection rates declined 53.5%, and metastatic prostate cancer rates increased 36.9%; all changes over time were statistically significant. We feel that the rates in our report are accurate and can be useful to providers and patients during the shared decision-making process surrounding prostate cancer screening.
Comparison with Previous Studies
Studies have consistently found that PSA screening has declined since the 2012 USPSTF statement [7,8,9,10,11]. Consistent with these reports, our study reported a decline in screening rates from 448.6 per 1000 person-years in 2010 to 334.0 per 1000 person-years in 2015. Drazer et al., using data from the National Health Interview Survey (NHIS), compared 2010 survey respondents with 2013 respondents and found a significant decline in PSA testing in men ages 50 to 59 from 33.2 to 24.8% and in men ages 60–74 from 51.2 to 43.6% [10]. These self-reported data may be subject to recall bias. Jemal et al. also used the NHIS data and compared screening rates in men 50–74 years of age between 2010 and 2013 and found that screening declined significantly from 36.8 to 29.9% [7]. The screening rate ratio of 0.81 is comparable with the present study. In contrast to the above studies, Kim et al. studied over 5 million men using administrative claims data and concluded that PSA screening rates remained stable from the years 2008 through 2013 in men under the age of 75 [11].
Prostate biopsy rates declined 64.3% in our study which is larger than previous reports. Review of self-reported case logs of urologists undergoing re-certification by the American Board of Urology showed a 28.7% relative decline in prostate biopsy when comparing years 2009–2012 with 2013–2016 [16]. In a study using administrative claims data of over five million men spanning the years 2005 to 2014, relative biopsy rates declined 33% [12]. A Medicare data set comparing 2012 with 2014 showed a 12.4% relative decline in biopsy rates [26]. As mentioned previously, the accuracy of rate calculations in these studies is questionable because of the challenge in identifying an accurate denominator.
Few studies have examined differences in rates of incident prostate cancer since the 2012 USPSTF Statement. Jemal et al. compared the rates of all incident cancers between 2011 and 2012 in men 50–74 years of age and found the rate ratio was 0.84 [7]. The decline was driven by local/regional disease for which the rate ratio was 0.83 while metastatic rates remained stable (rate ratio = 0.99). The incidence rate of localized and regional disease further declined from 2012 to 2013 (356.5 to 335.4 per 100,000, IRR = 0.94) yet incident rates of metastatic disease were unchanged [17]. We also observed relatively stable rates of localized and metastatic prostate cancer through 2013. However, using data that extended through 2015, we observed a decline in incident cancer detection yet an increase in metastatic disease. Negoita et al. noted a rise of distant disease rates with an inflection point around the years 2010–2011 [20]. However, the age group included men up to age 75 and it is possible this was driven by higher metastatic rates in men ages 70–75, who are at greater risk. Kelly et al. using SEER data, reported an increase in metastatic rates in men aged 45–54 and men aged 55–69 in the years 2012 to 2014 but interestingly also noted an increased rate in the years 2008 and 2011 [21]. These authors had no data on PSA screening rates in this study. In contrast, we saw relatively stable metastatic rates in the years 2010–2012. To our knowledge, we are the first study to report an increase in metastatic disease in men under the age of 70, the population most relevant for counseling in shared decision-making and to policy makers.
Strengths and Limitations
We could define an “eligible population” which served as the denominator in rate calculations, thus accurate rates could be determined. Our sample size was quite large and diverse, and PSA screening and biopsy rates were determined by the PSA test and biopsy result recorded in electronic medical records, not by patient recall or administrative claims data or order history. We must emphasize that the changes in our screening rates are associated with the timing of the 2012 USPSTF Statement but we cannot prove causality in this observational study.
To be a confounder, a variable must be associated with both the exposure and the outcome. Our exposure in this study was the time period; thus a confounder would need to be associated with time as well as with the outcome. We looked at several possible confounders in our analysis including age, race, comorbidities and care utilization; Table 1 shows minimal changes in these variables over the study time period. A widely used criterion to determine confounding is that a confounder must change the effect estimate by at least 10% [27]. When we adjusted our cancer detection rates and metastatic rates for age and race, we found negligible changes (0 to 3%) in estimated incidence rates, far below the 10% threshold. It is possible that other unmeasured confounders exist, although they would need sufficiently strong changes over the study time period to meet the criteria for confounding. Screening and biopsy rates were presented unadjusted, consistent with previous studies [7,8,9,10,11,12, 16, 25].
Another limitation of our study was that we did not use family history of prostate cancer in defining our eligible population as this data element was not uniformly collected in our health record. However, we have no reason to suspect that the distribution of family history would have changed during the study period.
It is important to recognize that some of the declines in screening, biopsy and incident cancer detection may be beneficial. False-positive PSA elevations result in unnecessary biopsies that are associated with inherent risks of the procedure along with patient anxiety and costs. Additionally, the detection of indolent cancers likely decreased although we were not able to quantify this outcome. Such an analysis was beyond the scope of this project as it would require detailed pathologic review including the number of biopsy cores positive, the extent of individual core length, and prostate size. The balance between finding fewer indolent cancers yet avoiding more metastatic disease warrants further study.
We do not have any comparative mortality data at this time but we hypothesize this stage migration may result in an increase in mortality yet additional time is needed to study this endpoint. In a recent large clinical trial, the median overall survival for men with metastatic prostate cancer was approximately six years (57.6 months), and longer in men with low-volume metastatic disease [28]. Also of interest will be to monitor screening, biopsy and detection rates following the 2018 USPSTF. We hypothesize that our screening rates may increase once again which will likely result in a higher incident cancer detection rate yet a lower metastatic rate.
Our study shows that in the years following the guideline change, major reductions in PSA screening have coincided with lower overall incident cancer detection rates yet higher rates of metastatic disease in men under the age of 70. The ideal screening strategy would identify only those cancers that require treatment. At present, this goal remains elusive.
References
American Cancer Society. Cancer Facts & Figures 2018. Atlanta: American Cancer Society; 2018.
Schröder FH, Hugosson J, Roobol MJ, et al; ERSPC Investigators. Screening and prostate cancer mortality: results of the European Randomised Study of Screening for Prostate Cancer (ERSPC) at 13 years of follow-up. Lancet 2014; 384(9959): 2027–2035.
Pinsky P, Prorok P, Yu K, et al. Extended mortality results for prostate cancer screening in the PLCO trial with median follow-up of 15 years. Cancer 2017; 123(4): 592–599.
Moyer VA; US Preventive Services Task Force. Screening for prostate cancer: US Preventive Services Task Force recommendation statement. Ann Intern Med 2012; 157(2):120–134.
Tasian GE, Cooperberg MR, Cowan JE, et al: Prostate specific antigen screening for prostate cancer: knowledge of attitudes towards, and utilization among primary care physicians. Urol Oncol 2012; 30(2): 155–160.
Grossman DC, US Preventive Services Task Force: Screening for prostate cancer: US Preventive Services Task Force recommendation statement. JAMA 2018; 319(18): 1901–1913.
Jemal A, Fedewa SA, Ma J, et al: Prostate cancer incidence and PSA testing patterns in relation to USPSTF screening recommendations. JAMA 2015; 314(19): 2054–2061.
Li J, Berkowitz Z and Hall IJ: Decrease in prostate cancer testing following the US Preventive Services Task Force (USPSTF) recommendations. J Am Board Fam Med 2015; 28(4): 491–493.
Sammon JD, Abdollah F, Choueiri TK, et al: Prostate-specific antigen screening after 2012 US Preventive Services Task Force recommendations. JAMA 2015; 314(19): 2077–2079.
Drazer MW, Huo D and Eggener SE: National prostate cancer screening rates after the 2012 US Preventive Services Task Force recommendations. J Clin Oncol 2015; 33(22): 2416–2423.
Kim SP, Kanes RJ, Gross CP, et al: Contemporary national trends of prostate cancer screening among privately insured men in the United States. Urology 2016; 97: 111–117.
Gershman B, Van Houten HK, Herrin J, et al: Impact of prostate-specific antigen (PSA) screening trials, and revised PSA screening guidelines on rates of prostate biopsy and postbiopsy complications. Eur Urol 2017; 71(1): 55–65.
Banerji JS, Wolff EM, Massman JD III, Odem-Davis K, Porter CR, Corman JM: Prostate needle biopsy outcomes in the era of the US Preventive Services Task Force recommendation against prostate specific antigen based screening. J Urol 2016; 195(1): 66–73.
Bhindi B, Mamdani M, Kulkarni GS, et al: Impact of the US Preventive Services Task Force recommendations against prostate specific antigen screening on prostate biopsy and cancer detection rates. J Urol 2015; 193(5): 1519–1524.
McGinley KF, McMahon GC and Brown GA: Impact of the US Preventive Services Task Force grade D recommendation: assessment of evaluations for elevated prostate-specific antigen and prostate biopsies in a large urology group practice following statement revision. Rev Urol 2015; 17(3): 171–177.
Halpern JA, Shoag JE, Artis AS, et al: National trends in prostate biopsy and radical prostatectomy volumes following the US preventive Services Task Force guidelines against prostate-specific antigen screening. JAMA Surg 2017; 152(2): 192–198.
Jemal A, Ma J, Siegel R, Fedewa S, Brawley O, Ward EM: Prostate cancer incidence rates 2 years after the US Preventive Services Task Force recommendations against screening. JAMA Oncol 2016; 2(12): 1657–1660.
Reese ACD, Wessel SR, Fisher SG, Mydlo JH: Evidence of prostate cancer “reverse stage migration” toward more advanced disease at diagnosis: data from the Pennsylvania Cancer Registry. Urol Oncol 2016; 34(8): 335.e21–335.e28.
Hu JC, Nguyen P, Mao J, et al: Increase in prostate cancer distant metastases at diagnosis in the United States. JAMA Oncol 2017; 3(5): 705–707.
Negoita S, Feuer EJ, Mariotto A, et al: Annual report to the nation on the status of cancer, part II: Recent changes in prostate cancer trends and disease characteristics. Cancer 2018; 124(13): 2801–2814.
Kelly SP, Anderson WF, Rosenberg PS, Cook MB: Past, current and future incidence rates and burden of metastatic prostate cancer in the United States. Eur Urol Focus 2018; (4): 121–127.
Escobar GJ, Gardner M, Greene JG, Draper D, Kipnis P: Risk-adjusting hospital mortality using a comprehensive electronic record in an integrated healthcare delivery system. Med Care 2013; 51: 446–453.
Amin MB, Edge SB, Greene FL, Byrd DR, Brookland RK eds. AJCC Cancer Staging Manual/Eighth Edition. New York, NY: Springer International Publishing; 2017.
Bernal JL, Cummins S, Gasparrini A: Interrupted time series regression for the evaluation of public health interventions: a tutorial. Int J Epidemiol 2017; 46(1): 348–355.
Harrington D, D’Agostino RB, Gatsonis C, et al: New guidelines for statistical reporting in the Journal. N Engl J Med 2019 381: 285–286.
Henry MA, Howard DH, Davies BJ, Filson CP: Variation in use of prostate biopsy following changes in prostate cancer screening guidelines. J Urol 2017; 198(5): 1046–1053.
Greenland S, Morgenstern H: Ecological bias, confounding, and effect modification. Int J Epidemiol 1989;18(1): 269–274.
Sweeney CJ, Chen YH, Carducci M, et al. Chemohormonal therapy in metastatic hormone-sensitive prostate cancer. N Engl J Med 2015; 373(8): 737–746.
Funding
The study was supported by The Permanente Medical Group Delivery Science and Physician Researcher Programs.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of Interest
The authors declare that they do not have a conflict of interest.
Disclaimer
This study was reviewed and approved by the Kaiser Permanente Northern California Institutional Review Board.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Prior Presentations
ASCO 2018 Genitourinary Cancers Symposium February 8, 2018.
Rights and permissions
About this article
Cite this article
Presti, J., Alexeeff, S., Horton, B. et al. Changes in Prostate Cancer Presentation Following the 2012 USPSTF Screening Statement: Observational Study in a Multispecialty Group Practice. J GEN INTERN MED 35, 1368–1374 (2020). https://doi.org/10.1007/s11606-019-05561-y
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11606-019-05561-y