ABSTRACT
BACKGROUND
The use of surrogate and composite endpoints, disease-specific mortality as an endpoint, and relative (rather than absolute) risk reporting in clinical trials may produce results that are misleading or difficult to interpret.
OBJECTIVE
To describe the prevalence of these endpoints and of relative risk reporting in medication trials.
DESIGN AND MAIN MEASURES
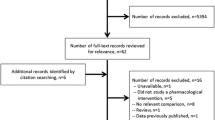
We analyzed all randomized medication trials published in the six highest impact general medicine journals between June 1, 2008 and September 30, 2010 and determined the percentage using these endpoints and the percentage reporting results in the abstract exclusively in relative terms.
KEY RESULTS
We identified 316 medication trials, of which 116 (37%) used a surrogate primary endpoint and 106 (34%) used a composite primary endpoint. Among 118 trials in which the primary endpoint involved mortality, 32 (27%) used disease-specific mortality rather than all-cause mortality. Among 157 trials with positive results, 69 (44%) reported these results in the abstract exclusively in relative terms. Trials using surrogate endpoints and disease-specific mortality as an endpoint were more likely to be exclusively commercially funded (45% vs. 29%, difference 15% [95% CI 5%–26%], P = 0.004, and 39% vs. 16%, difference 22% [95% CI 6%-37%], P = 0.007, respectively). Trials using surrogate endpoints were more likely to report positive results (66% vs. 49%, difference 17% [95% CI 5%–28%], P = 0.006) while those using mortality endpoints were less likely to be positive (46% vs. 62%, difference −16% [95% CI −27%–−4%], P = 0.01).
CONCLUSIONS
The use of surrogate and composite endpoints, endpoints involving disease-specific mortality, and relative risk reporting is common. Articles should highlight the limitations of these endpoints and should report results in absolute terms.
Similar content being viewed by others
References
Kraemer HC, Frank E. Evaluation of comparative treatment trials: assessing clinical benefits and risks for patients, rather than statistical effects on measures. JAMA. 2010;304(6):683–4.
Krumholz HM, Lee TH. Redefining quality–implications of recent clinical trials. N Engl J Med. 2008;358(24):2537–9.
Bero L, Oostvogel F, Bacchetti P, Lee K. Factors associated with findings of published trials of drug-drug comparisons: why some statins appear more efficacious than others. PLoS Med. 2007;4(6):e184.
Ridker PM, Torres J. Reported outcomes in major cardiovascular clinical trials funded by for-profit and not-for-profit organizations: 2000–2005. JAMA. 2006;295(19):2270–4.
la Cour JL, Brok J, Gøtzsche PC. Inconsistent reporting of surrogate outcomes in randomised clinical trials: cohort study. BMJ. 2010;341:c3653.
Fleming TR, DeMets DL. Surrogate end points in clinical trials: are we being misled? Ann Intern Med. 1996;125(7):605–13.
Freemantle N, Calvert M, Wood J, Eastaugh J, Griffin C. Composite outcomes in randomized trials: greater precision but with greater uncertainty? JAMA. 2003;289(19):2554–9.
Yeh RW, Go AS. Rethinking the epidemiology of acute myocardial infarction: challenges and opportunities. Arch Intern Med. 2010;170(9):759–64.
Ferreira-González I, Busse JW, Heels-Ansdell D, Montori VM, Akl EA, Bryant DM, Alonso-Coello P, Alonso J, Worster A, Upadhye S, Jaeschke R, Schünemann HJ, Permanyer-Miralda G, Pacheco-Huergo V, Domingo-Salvany A, Wu P, Mills EJ, Guyatt GH. Problems with use of composite end points in cardiovascular trials: systematic review of randomised controlled trials. BMJ. 2007;334(7597):786.
Freemantle N, Calvert MJ. Interpreting composite outcomes in trials. BMJ. 2010;341:c3529.
Cordoba G, Schwartz L, Woloshin S, Bae H, Gøtzsche PC. Definition, reporting, and interpretation of composite outcomes in clinical trials: systematic review. BMJ. 2010;341:c3920.
Black WC, Haggstrom DA, Welch HG. All-cause mortality in randomized trials of cancer screening. J Natl Cancer Inst. 2002;94(3):167–73.
Laupacis A, Sackett DL, Roberts RS. An assessment of clinically useful measures of the consequences of treatment. N Engl J Med. 1988;318:1728–33.
Nuovo J, Melnikow J, Chang D. Reporting number needed to treat and absolute risk reduction in randomized controlled trials. JAMA. 2002;287(21):2813–4.
Marshall KG. Prevent: how much harm? How much benefit? 1. Influence of reporting methods on perception of benefits. CMAJ. 1996;154(10):1493–9.
Lim E, Brown A, Helmy A, Mussa S, Altman DG. Composite outcomes in cardiovascular research: a survey of randomized trials. Ann Intern Med. 2008;149(9):612–7.
Kip KE, Hollabaugh K, Marroquin OC, Williams DO. The problem with composite end points in cardiovascular studies: the story of major cardiac events and percutaneous coronary intervention. J Am Coll Cardiol. 2008;51(7):701–7.
Dryver E, Hux JE. Reporting of numerical and statistical differences in abstracts: improving but not optimal. J Gen Intern Med. 2002;17(3):203–6.
Schwartz LM, Woloshin S, Dvorin EL, Welch HG. Ratio measures in leading medical journals: structured review of accessibility of underlying absolute risks. BMJ. 2006;333(7581):1248.
Hochman M, McCormick D. Characteristics of published comparative effectiveness studies of medications. JAMA. 2010;303(10):951–8.
Science journal citation reports ISI: Summer 2008. Thomson Reuters Web site. http://scientific.thomson.com/products/jcr/. Accessed July 5, 2011.
Bucher HC, Guyatt GH, Cook DJ, Holbrook A, McAlister FA. How to use an article measuring the effect of an intervention on surrogate end points. JAMA. 1999;282(8):771–8.
Temple RJ. A regulatory authority’s opinion about surrogate endpoints. In: Nimmo WS, Tucker GT, eds. Clinical Measurement in Drug Evaluation. New York: Wiley; 1995.
Grady D, Redberg RF. Less is more: how less health care can result in better health. Arch Intern Med. 2010;170(9):749–50.
Rosen C. Revisiting the rosiglitazone story – lessons learned. NEJM. 2010;363(9):803–5.
Als-Nielsen B, Chen W, Gluud C, Kjaergard LL. Association of funding and conclusions in randomized drug trials: a reflection of treatment effect or adverse events? JAMA. 2003;290(7):921–8.
Kjaergard LL, Als-Nielsen B. Association between competing interests and authors’ conclusions: epidemiological study of randomised clinical studies published in the BMJ. BMJ. 2002;325(7358):249–52.
Bekelman JE, Li Y, Gross CP. Scope and impact of financial conflicts of interest in biomedical research: a systematic review. JAMA. 2003;289(4):454–65.
Tomlinson G, Detsky AS. Composite endpoints in randomized trials: there is no free lunch. JAMA. 2010;303(3):267–8.
Guyatt G, Rennie D, Meade MO, Cook DJ. Can one be confident that the component endpoints share similar relative risk reductions? Users’ guides to the medical literature. Chapter 10.4. Composite Endpoints.
Montori VM, Permanyer-Miralda G, Ferreira-González I, Busse JW, Pacheco-Huergo V, Bryant D, Alonso J, Akl EA, Domingo-Salvany A, Mills E, Wu P, Schünemann HJ, Jaeschke R, Guyatt GH. Validity of composite endpoints in clinical trials. BMJ. 2005;330(7491):594–6.
Guyatt GH, Sackett DL, Cook DJ. Users’ guides to the medical literature. II. How to use an article about therapy or prevention. A. Are the results of the study valid? JAMA. 1993;270(21):2598–601.
Contributors
We have listed everyone who made substantial contributions to this analysis as authors of the article.
Funders
This study was unfunded (i.e. it received no internal or external funding).
Prior Presentations
This work was presented as a poster at the 2011 Society of General Internal Medicine National Meeting in Phoenix, Arizona.
Conflict of Interest
None disclosed.
Author information
Authors and Affiliations
Corresponding author
Additional information
The views expressed in this article are solely those of the author and do not necessarily represent those of the Department of Veterans Affairs or of the Robert Wood Johnson Foundation
Electronic supplementary material
Below is the link to the electronic supplementary material.
ESM 1
(DOC 44 kb)
Rights and permissions
About this article
Cite this article
Hochman, M., McCormick, D. Endpoint Selection and Relative (Versus Absolute) Risk Reporting in Published Medication Trials. J GEN INTERN MED 26, 1246–1252 (2011). https://doi.org/10.1007/s11606-011-1813-7
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11606-011-1813-7