Abstract
Purpose
To evaluate the associations between comorbidities and kidney function decline at 6-month and 1-year follow-up in outpatients with initial estimated glomerular filtration rate (eGFR) ≥ 30 mL/min/1.73 m2.
Materials and methods
Outpatients aged 18 and older with confirmed diagnosis, who had eGFR ≥ 30 mL/min/1.73 m2 measured between April 2017 and March 2019, were included in this retrospective observational study. Of them, 30,595 included outpatients had 6-month eGFR test and 27,698 included outpatients had 1-year eGFR test. The outpatients were further divided into two groups based on initial eGFR: between 30 and 59 and ≥ 60 mL/min/1.73 m2. Impaired renal function was defined as eGFR declined to below 30 mL/min/1.73 m2. The comorbidities with P values less than 0.1 identified in univariable logistic regression models were entered into the multivariable analysis with backward selection, thereby identifying comorbidities that increased the risk of eGFR decline at 6-month and 1-year follow-up.
Results
Outpatients with initial eGFR between 30 and 59 mL/min/1.73 m2 were 175.94 times more likely to have eGFR decline at 6 months, and were 94.10 times more likely to have eGFR decline at 1 year, compared with their corresponding initial eGFR ≥ 60 counterparts. Multivariable logistic regression analyses disclosed that chronic kidney disease, hypertension, and heart failure were independent risk factors for eGFR decline in outpatients with initial eGFR between 30 and 59 mL/min/1.73 m2.
Conclusions
Outpatients with initial eGFR ≥ 60 mL/min/1.73 m2 might not need routine eGFR test prior to contrast-enhanced CT scan for 1 year. In addition, chronic kidney disease, hypertension, and heart failure increased the risk of declined renal function, particularly, in outpatients with initial eGFR between 30 and 59 mL/min/1.73 m2.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Computed tomography (CT) using iodine-containing contrast media is a routine and highly informative diagnostic imaging technique. Most of the administered iodinated contrast media is excreted from the kidneys. However, post-contrast acute kidney injury (PC-AKI), one of the most serious adverse reactions to contrast media [1, 2], may occurred in some patients, particularly in patients with kidney disease [3,4,5,6]. PC-AKI often presents within 2 to 5 days after administration of contrast media, with an increase in the serum creatinine (sCr) level by 0.5 mg/dL or more (or 25% elevation or more) [1, 7]. The risk factors for PC-AKI may be procedure-related or patient-related [2]. And patient-related risk factors include aging, lower estimated glomerular filtration rate (eGFR), acute renal failure, chronic kidney disease (CKD), and congestive heart failure [2, 5, 8, 9].
In patients with eGFR less than 30 mL/min/1.73 m2, further deterioration of renal function may occur after contrast-enhanced CT scans [10]. A propensity score matching study concluded that the likelihood of PC-AKI increased by 51% in adult patients with pre-CT eGFR ≤ 30 mL/min/1.73 m2 [11]. Japanese guidelines recommend that patients with eGFR < 30 mL/min/1.73 m2 should not receive iodinated contrast media without appropriate precautions (e.g. using physiological saline intravenously) [5]. The European Society for Urogenital Radiology (ESUR) classifies three levels of renal dysfunction: eGFR < 60 mL/min/1.73 m2, eGFR < 45 mL/min/1.73 m2, and eGFR < 30 mL/min/1.73 m2; the last two levels are risk factors for PC-AKI [1, 2]. For patients with acute disease, patients with acute deterioration of chronic disease, or hospitalized patients, ESUR recommends evaluating renal function within 7 days before contrast media administration [1, 2]. In contrast, ESUR recommends that all other patients need to evaluate renal function within 3 months before contrast media administration [1, 2]. The American College of Radiology (ACR) also proposes that individual patient’s condition and associated risk factor(s) should be taken into consideration before using contrast media [3]. Therefore, in order to identify patients at high risk of PC-AKI, the measurement of eGFR, a hematologic marker of renal function, is suggested to be implemented before the administration of contrast media [1, 2, 5, 12, 13]. However, few studies have evaluated renal function over time, and the timing of eGFR test prior to contrast-enhanced CT has not been standardized.
The purpose of this retrospective observational study was to identify patient-related risk factors for impaired renal function at 6 months and 1 year. Thus, we retrospectively reviewed the variation in eGFR over time in outpatients, and selected outpatients whose eGFR values were decreased from ≥ 30 to < 30 mL/min/1.73 m2 at 6 months or 1 year after the initial eGFR evaluation. Univariable and multivariable logistic regression analyses were then performed to evaluate the associations between comorbidities and eGFR decline, thereby identifying independent risk factors for eGFR decline. The anticipated results of this study may provide insight into the timing of eGFR test prior to contrast-enhanced CT for patients with various comorbidities.
Materials and Methods
Study population
This retrospective observational study was reviewed and approved by the Institutional Review Board of our hospital (IRB approval number: 20–196). The requirement for signed informed consent was waived because of the retrospective nature of this study.
Outpatients who visited our hospital and underwent eGFR assessment, with or without contrast-enhanced CT, from April 2017 through March 2019 were initially included. Because the assessment of renal function differs between children and adults, outpatients younger than 18 years were excluded from the study. Regarding the selection of diseases of interest, we referred to guidelines for the use of contrast media [5] and chose diseases that are generally considered to cause renal dysfunction [1, 2, 14,15,16,17,18,19] or whose treatments are associated with renal dysfunction [20]. However, since most included outpatients had multiple comorbidities, all comorbidities found in included outpatients were documented in this study. The baseline disease characteristics of all included outpatient were extracted from medical records. And all included patients were outpatients at the baseline, 6-month follow-up, and 1-year follow-up.
Kidney function evaluation
Outpatients’ diagnostic and renal function data were extracted from the clinical data warehouse of our hospital. Renal function was assessed based on the eGFR that was calculated using an equation specific for Japanese [21]. According to the classification of CKD [1, 2], outpatients were divided into three groups based on the initial eGFR: ≥ 60, between 30 and 59, and < 30 mL/min/1.73 m2. Outpatients with an initial eGFR of < 30 mL/min/1.73 m2 were excluded, because they already had impaired renal function and needed appropriate precaution for contrast-enhanced CT [1, 2, 5]. In this study, decline in renal function was defined as when eGFR dropped to below 30 mL/min/1.73 m2 at 6 months or 1 year after the initial evaluation.
Statistical analysis
Age is presented as mean and standard deviation; the rest variables are presented as count and percentage. The differences between two initial eGFR groups were examined with the Fisher’s exact test, except for the difference in age that was examined with the independent two samples test. The association between initial eGFR value and eGFR decline (below 30 mL/min/1.73 m2) was evaluated by univariable logistic regression analysis. Subsequently, univariable logistic regression analyses were performed to evaluate the associations between comorbidity and eGFR decline within the initial eGFR group at 6 months and 1 year. The comorbidities with P values less than 0.1 identified in univariable logistic regression models were chosen to be entered into the multivariable analysis with backward selection. All statistical hypothesis tests were two-side, and the significance level was set as 0.05. The statistical analyses were performed using the IBM SPSS Statistics 25.0 (IBM Corporation, Armonk, NY, USA).
Results
Patient selection
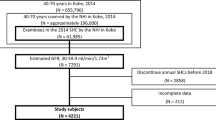
A total of 117,019 outpatients aged 18 years and older who underwent renal function test between April 2017 and March 2019 were initially selected for this retrospective study. The flowchart of outpatient selection is shown in Fig. 1. Of them, 19,020 outpatients who had no confirmed disease diagnosis and 1,009 outpatients who had initial eGFR < 30 mL/min/1.73m2 were excluded. As a result, 96,990 outpatients were included. After excluding 66,395 outpatients who did not have eGFR test at 6-month follow-up, the remaining 30,595 outpatients who had eGFR test at 6 months were subjected to the final analysis. In addition, after excluding 69,292 outpatients who did not have eGFR test at 1-year follow-up, the remaining 27,698 outpatients who had eGFR test at 1 year were independently analyzed in this retrospective study (Fig. 1).
Baseline demographic and clinical characteristics
Among 30,595 outpatients with 6-month follow-up data, 5,686 outpatients had initial eGFR between 30 and 59 mL/min/1.73 m2, and 24,909 outpatients had initial eGFR ≥ 60 mL/min/1.73 m2 (Table 1). Outpatients with initial eGFR between 30 and 59 mL/min/1.73 m2 were significantly older than those with initial eGFR ≥ 60 mL/min/1.73 m2 (P < 0.001). There were significantly more males in the initial eGFR between 30 and 59 group compared with the initial eGFR ≥ 60 group (P < 0.001). Outpatients with initial eGFR between 30 and 59 mL/min/1.73 m2 had significant higher percentages of CKD, diabetes mellitus (DM), hypertension, acquired absence of kidney, glomerular diseases, gout or hyperuricemia, ischemic heart diseases, arrhythmia, heart failure, arteriosclerosis obliterans, hyperlipidemia, chronic obstructive pulmonary disease, cerebral infarction, gastric ulcer, IgG4-related disease, hyperthyroidism or hypothyroidism, migraine, asthma, malignant neoplasm of colon, and malignant neoplasms of urinary tract, than those with initial eGFR ≥ 60 mL/min/1.73 m2 (all p ≤ 0.03) (Table 1).
Among 27,698 outpatients with 1-year follow-up data, 4,954 outpatients had initial eGFR between 30 and 59 mL/min/1.73 m2, and 22,744 outpatients had initial eGFR ≥ 60 mL/min/1.73 m2 (Table 2). Outpatients with initial eGFR between 30 and 59 mL/min/1.73 m2 were significantly older than those with initial eGFR ≥ 60 mL/min/1.73 m2 (P < 0.001). There were significantly more males in the initial eGFR between 30 and 59 group compared with the initial eGFR ≥ 60 group (P < 0.001). Outpatients with initial eGFR between 30 and 59 mL/min/1.73 m2 had significant higher percentages of CKD, DM, hypertension, acquired absence of kidney, glomerular diseases, gout or hyperuricemia, ischemic heart diseases, arrhythmia, heart failure, arteriosclerosis obliterans, hyperlipidemia, chronic obstructive pulmonary disease, cerebral infarction, gastric ulcer, IgG4-related disease, hyperthyroidism or hypothyroidism, migraine, asthma, malignant neoplasm of colon, and malignant neoplasms of urinary tract, than those with initial eGFR ≥ 60 mL/min/1.73 m2 (all p ≤ 0.011) (Table 2).
Association between initial eGFR and eGFR decline
In outpatients with 6-month follow-up data, 3.4% of outpatients with initial eGFR between 30 and 59 mL/min/1.73 m2 had eGFR decline, defined as eGFR decreasing to below 30 mL/min/1.73 m2, at 6 months. In contrast, only 0.02% of outpatients with initial eGFR ≥ 60 mL/min/1.73 m2 had eGFR decline at 6 months (Table 3). Univariable logistic regression analysis revealed that outpatients with initial eGFR between 30 and 59 mL/min/1.73 m2 were 175.94 times more likely to have eGFR decline at 6 months, compared to those with initial eGFR ≥ 60 mL/min/1.73 m2 (P < 0.001) (Table 3).
In outpatients with 1-year follow-up data, 5.1% of outpatients with initial eGFR between 30 and 59 mL/min/1.73 m2 had eGFR decline at 1 year. In contrast, only 0.06% of outpatients with initial eGFR ≥ 60 mL/min/1.73 m2 had eGFR decline at 1 year (Table 3). Univariable logistic regression analysis indicated that outpatients with initial eGFR between 30 and 59 mL/min/1.73 m2 were 94.10 times more likely to have eGFR decline at 1 year, compared to those with initial eGFR ≥ 60 mL/min/1.73 m2 (P < 0.001) (Table 3).
Associations between comorbidities and eGFR decline in outpatients with initial eGFR between 30 and 59 mL/min/1.73 m2
Univariable logistic regression analysis revealed that in outpatients with initial eGFR between 30 and 59 mL/min/1.73 m2, CKD, DM, hypertension, glomerular diseases, gout or hyperuricemia, arrhythmia, and heart failure were significantly associated with eGFR decline at 6-month follow-up (all p ≤ 0.011) (Table 4). Moreover, in outpatients with initial eGFR between 30 and 59 mL/min/1.73 m2, CKD, DM, hypertension, glomerular diseases, gout or hyperuricemia, arrhythmia, heart failure, cerebral infarction, migraine, and malignant neoplasm of urinary tract were significantly associated with eGFR decline at 1-year follow-up (all p ≤ 0.024) (Table 5).
Associations between comorbidities and eGFR decline in outpatients with initial eGFR ≥ 60 mL/min/1.73 m2
Univariable logistic regression analysis disclosed that in outpatients with initial eGFR ≥ 60 mL/min/1.73 m2, CKD and heart failure were significantly associated with eGFR decline at 6-month follow-up (both p ≤ 0.036) (Supplementary Table 1). Furthermore, in outpatients with initial eGFR ≥ 60 mL/min/1.73 m2, hypertension, gout or hyperuricemia, ischemic heart diseases, arrhythmia, heart failure, and myasthenia gravis were significantly associated with eGFR decline at 1-year follow-up (all p ≤ 0.045) (Supplementary Table 2).
Independent risk factors for eGFR decline identified by multivariable logistic regression analysis
To investigate whether the comorbidities had independent and significant influence on the risk of eGFR declined to below 30, four multivariable logistic regression models were conducted. The comorbidities with P values less than 0.1 in Tables 4, 5 and Supplementary Tables 1, 2 were entered into the process of model selection for the corresponding multivariable logistic regression analysis.
Multivariable logistic regression analysis revealed that in outpatients with initial eGFR between 30 and 59 mL/min/1.73 m2, CKD, hypertension, and heart failure were significant risk factors for eGFR decline at 6-month follow-up (all p ≤ 0.034) (Table 6). However, more comorbidities, including CKD, hypertension, glomerular diseases, gout or hyperuricemia, heart failure, migraine, and malignant neoplasm of urinary tract, were significant risk factors for eGFR decline at 1-year follow-up in outpatients with initial eGFR between 30 and 59 mL/min/1.73 m2 (all p ≤ 0.014) (Table 6).
On the other hand, multivariable logistic regression analysis disclosed that in outpatients with initial eGFR ≥ 60 mL/min/1.73 m2, only CKD was significant risk factor for eGFR decline at 6-month follow-up (P < 0.001) (Table 6). Moreover, hypertension and heart failure significant risk factors for eGFR decline at 1-year follow-up in outpatients with initial eGFR ≥ 60 mL/min/1.73 m2 (both p ≤ 0.010) (Table 6).
Associations between contrast administration and eGFR decline in outpatients with initial eGFR between 30 and 59 mL/min/1.73 m2
As revealed in Table 3, outpatients with initial eGFR between 30 and 59 mL/min/1.73 m2 were at significantly increased risk of eGFR decline at 6 months and 1 year (OR = 175.94 and 94.10, respectively). So, the associations between contrast administration and eGFR decline, regardless of comorbidities, were assessed in outpatients with initial eGFR between 30 and 59 mL/min/1.73 m2. Logistic regression analysis revealed that contrast administration was not significantly associated with eGFR decline in outpatients receiving eGFR test at 6 months (OR = 1.03, P = 0.886) (Supplementary Table 3). And contrast administration was also not significantly associated with eGFR decline in outpatients receiving eGFR test at 1 year (OR = 0.75, P = 0.100) (Supplementary Table 3).
Discussion
In the present retrospective observational study, 30,595 outpatients with eGFR test at 6 months and 27,698 outpatients with eGFR test at 1 year were independently analyzed. The results indicated that at both 6 months and 1 year, outpatients with initial eGFR between 30 and 59 mL/min/1.73 m2 were older and had higher percentages of comorbidities, than their corresponding initial eGFR ≥ 60 counterparts. Incidence rates of eGFR decline at 6 months and 1 year were 0.02% and 0.06%, respectively, in outpatients with initial eGFR ≥ 60 mL/min/1.73 m2, but were 3.4% and 5.1%, respectively, in outpatients with initial eGFR between 30 and 59 mL/min/1.73 m2. Outpatients with initial eGFR between 30 and 59 mL/min/1.73 m2 were 175.94 times more likely to have eGFR decline at 6 months, and were 94.10 times more likely to have eGFR decline at 1 year, compared with their corresponding initial eGFR ≥ 60 counterparts. Multivariable logistic regression analysis disclosed that in outpatients with initial eGFR between 30 and 59 mL/min/1.73 m2, CKD, hypertension, and heart failure were independent risk factors for eGFR decline at 6 months. In addition to the above-mentioned comorbidities, glomerular diseases, gout or hyperuricemia, migraine, and malignant neoplasm of urinary tract were also independent risk factors for eGFR decline at 1 year for outpatients with initial eGFR between 30 and 59 mL/min/1.73 m2. On the contrary, in outpatients with initial eGFR ≥ 60 mL/min/1.73 m2, only CKD was independent risk factor for eGFR decline at 6 months, and both hypertension and heart failure were independent risk factors for eGFR decline at 1 year.
Current findings suggested that the vast majority of outpatients with initial eGFR ≥ 60 mL/min/1.73 m2 still have sufficient renal function to receive contrast-enhanced CT scan at 6 month and 1 year, and that annual renal function reassessment prior to the use of contrast media may be suitable for such outpatients. In hospitalized children with pre-CT eGFR ≥ 60 mL/min/1.73 m2, iodinated contrast media was not associated with PC-AKI [22]. On the other hand, it is widely acknowledged that renal function declines with age [23, 24], and we also found outpatients with lower initial eGFR were older.
Outpatients with initial eGFR between 30 and 59 mL/min/1.73 m2 had higher incidence of almost all comorbidities documented in this study. Of them, outpatients with CKD, hypertension, or heart failure were more likely to have eGFR decline at 6 months and 1 year. Our findings were consistent with the results of previous studies [1, 2, 14,15,16,17,18,19], and a questionnaire survey study conducted in Japan, which concluded that chronic and acute kidney diseases (96.7% and 93.6%, respectively) were common risk factors for PC-AKI in Japan [25].
Age, sex, and sCr are considered in the current formula-based calculation of eGFR [26]. However, the concentration of sCr is altered by diurnal variation, menstrual cycle, nutritional status, and muscle mass to some degree [27]. A recent meta-analysis evaluated the influence of the within-subject biological variation of sCr on the reliability of eGFR, and concluded that eGFR can discriminate between true change in kidney function and random fluctuation [28]. Thus, despite biological variation of sCr, eGFR is a reliable tool for the evaluation of kidney function [28, 29].
On the other hand, gadolinium (Gd)-based contrast media are often used for contrast-enhanced magnetic resonance imaging [30], but are associated with tissue retention of Gd and nephrogenic systemic fibrosis [31, 32]. On top of that, contrast media extravasation may further worsen the impact of contrast media to patients [33]. Multiple lines of evidence suggested that patients with an eGFR of < 30 mL/min/1.73 m2 should not receive Gd-based contrast media without appropriate precautions [34, 35]. A questionnaire survey study revealed that in Japan, eGFR test was most frequently performed prior to the use of iodinated and Gd-based contrast media (80.8% and 82.6%, respectively) [25].
Several limitations of this study needed to be addressed. First of all, we extracted the disease diagnosis information according to the diagnoses entered into the database. In actual clinical practice, some diagnoses might reflect the wordings required by insurance companies; therefore, the possibility of outpatient misclassification cannot be ruled out. Second, although all included patients were outpatients at the baseline, 6-month follow-up, and 1-year follow-up, whether the included outpatients were hospitalized during the study period was not documented in this study. Third, the data analyzed were from a single institution, indeed a university hospital (an academic medical center). Generally speaking, patients admitted to a university hospital have more severe conditions than those admitted to general medical institutions. Hence, the incidence of renal function decline over the study period might be overestimated in this study, compared to the nationwide incidence. Fourth, most included outpatients had multiple comorbidities, and all comorbidities found in included outpatients were documented in this study. A huge variety of medicines were taken by the included outpatients. On top of that, dosage and frequency would further complicate the analysis of medicines, exceeding the word count limit. Further research is warranted to investigate the influence of medicines for CKD, hypertension, or heart failure on eGFR decline.
In conclusion, both renal function and comorbidity at the initial eGFR examination should be considered to deduce the appropriate timing regarding renal function evaluation before the use of contrast media. Outpatients with initial eGFR ≥ 60 mL/min/1.73 m2 were much less likely to have eGFR decline at 6 months and 1 year, compared with their corresponding initial eGFR between 30 and 59 counterparts. Therefore, outpatients with initial eGFR ≥ 60 mL/min/1.73 m2 might not need routine eGFR test prior to contrast-enhanced CT scan. Furthermore, CKD, hypertension, and heart failure were main risk factors for renal function decline. If outpatients were diagnosed with CKD, hypertension, or heart failure, close monitoring eGFR might be necessary, particularly, in outpatients with initial eGFR between 30 and 59 mL/min/1.73 m2.
References
van der Molen AJ, Reimer P, Dekkers IA, Bongartz G, Bellin MF, Bertolotto M, et al. Post-contrast acute kidney injury - part 1: definition, clinical features, incidence, role of contrast medium and risk factors : recommendations for updated ESUR contrast medium safety committee guidelines. Eur Radiol. 2018;28:2845–55.
van der Molen AJ, Reimer P, Dekkers IA, Bongartz G, Bellin MF, Bertolotto M, et al. Post-contrast acute kidney injury. Part 2: risk stratification, role of hydration and other prophylactic measures, patients taking metformin and chronic dialysis patients : recommendations for updated ESUR contrast medium safety committee guidelines. Eur Radiol. 2018;28:2856–69.
Davenport MS, Perazella MA, Yee J, Dillman JR, Fine D, McDonald RJ, et al. Use of intravenous iodinated contrast media in patients with kidney disease: consensus statements from the American college of radiology and the national kidney foundation. Radiology. 2020;294:660–8.
Kene M, Arasu VA, Mahapatra AK, Huang J, Reed ME. Acute kidney injury after ct in emergency patients with chronic kidney disease: a propensity score-matched analysis. West J Emerg Med. 2021;22:614–22.
Isaka Y, Hayashi H, Aonuma K, Horio M, Terada Y, Doi K, et al. Guideline on the use of iodinated contrast media in patients with kidney disease 2018. Jpn J Radiol. 2020;38:3–46.
Wu MJ, Tsai SF. Patients with different stages of chronic kidney disease undergoing intravenous contrast-enhanced computed tomography-the incidence of contrast-associated acute kidney injury. Diagnostics (Basel). 2022;12:864.
Mehran R, Dangas GD, Weisbord SD. Contrast-associated acute kidney injury. N Engl J Med. 2019;380:2146–55.
Lameire N, Adam A, Becker CR, Davidson C, McCullough PA, Stacul F, et al. Baseline renal function screening. Am J Cardiol. 2006;98:21K-K26.
Toprak O. Conflicting and new risk factors for contrast induced nephropathy. J Urol. 2007;178:2277–83.
Davenport MS, Khalatbari S, Cohan RH, Dillman JR, Myles JD, Ellis JH. Contrast material-induced nephrotoxicity and intravenous low-osmolality iodinated contrast material: risk stratification by using estimated glomerular filtration rate. Radiology. 2013;268:719–28.
Gorelik Y, Bloch-Isenberg N, Yaseen H, Heyman SN, Khamaisi M. Acute kidney injury after radiocontrast-enhanced computerized tomography in hospitalized patients with advanced renal failure: a propensity-score-matching analysis. Invest Radiol. 2020;55:677–87.
Bjallmark A, Bazzi M, Karlsson M, Krakys E, Kihlberg J. Radiology departmental policy compliance with Swedish guidelines regarding post-contrast acute kidney injury for examinations with iodinated contrast media. Radiography (Lond). 2021;27:1058–63.
Herts BR, Schneider E, Poggio ED, Obuchowski NA, Baker ME. Identifying outpatients with renal insufficiency before contrast-enhanced CT by using estimated glomerular filtration rates versus serum creatinine levels. Radiology. 2008;248:106–13.
Badve SV, Pascoe EM, Tiku A, Boudville N, Brown FG, Cass A, et al. Effects of allopurinol on the progression of chronic kidney disease. N Engl J Med. 2020;382:2504–13.
Damman K, Navis G, Voors AA, Asselbergs FW, Smilde TD, Cleland JG, et al. Worsening renal function and prognosis in heart failure: systematic review and meta-analysis. J Card Fail. 2007;13:599–608.
Mallamaci F, Tripepi G. Blood pressure variability in chronic kidney disease patients. Blood Purif. 2013;36:58–62.
Obermayr RP, Temml C, Gutjahr G, Knechtelsdorfer M, Oberbauer R, Klauser-Braun R. Elevated uric acid increases the risk for kidney disease. J Am Soc Nephrol. 2008;19:2407–13.
Rudnick M, Feldman H. Contrast-induced nephropathy: what are the true clinical consequences? Clin J Am Soc Nephrol. 2008;3:263–72.
Testani JM, Coca SG, McCauley BD, Shannon RP, Kimmel SE. Impact of changes in blood pressure during the treatment of acute decompensated heart failure on renal and clinical outcomes. Eur J Heart Fail. 2011;13:877–84.
Usui J, Yamagata K, Imai E, Okuyama H, Kajiyama H, Kanamori H, et al. Clinical practice guideline for drug-induced kidney injury in Japan 2016: digest version. Clin Exp Nephrol. 2016;20:827–31.
Matsuo S, Imai E, Horio M, Yasuda Y, Tomita K, Nitta K, et al. Revised equations for estimated GFR from serum creatinine in Japan. Am J Kidney Dis. 2009;53:982–92.
Gilligan LA, Davenport MS, Trout AT, Su W, Zhang B, Goldstein SL, et al. Risk of acute kidney injury following contrast-enhanced CT in hospitalized pediatric patients: a propensity score analysis. Radiology. 2020;294:548–56.
Nitta K, Okada K, Yanai M, Takahashi S. Aging and chronic kidney disease. Kidney Blood Press Res. 2013;38:109–20.
Toyama T, Kitagawa K, Oshima M, Kitajima S, Hara A, Iwata Y, et al. Age differences in the relationships between risk factors and loss of kidney function: a general population cohort study. BMC Nephrol. 2020;21:477.
Tsushima Y, Ishiguchi T, Murakami T, Hayashi H, Hayakawa K, Fukuda K, et al. Safe use of iodinated and gadolinium-based contrast media in current practice in Japan: a questionnaire survey. Jpn J Radiol. 2016;34:130–9.
Mula-Abed WA, Al Rasadi K, Al-Riyami D. Estimated glomerular filtration rate (eGFR): a serum creatinine-based test for the detection of chronic kidney disease and its impact on clinical practice. Oman Med J. 2012;27:108–13.
Samra M, Abcar AC. False estimates of elevated creatinine. Perm J. 2012;16:51–2.
Thoni S, Keller F, Denicolo S, Buchwinkler L, Mayer G. Biological variation and reference change value of the estimated glomerular filtration rate in humans: a systematic review and meta-analysis. Front Med (Lausanne). 2022;9:1009358.
Hilderink JM, van der Linden N, Kimenai DM, Litjens EJR, Klinkenberg LJJ, Aref BM, et al. Biological variation of creatinine, cystatin C, and eGFR over 24 hours. Clin Chem. 2018;64:851–60.
Fraum TJ, Ludwig DR, Bashir MR, Fowler KJ. Gadolinium-based contrast agents: a comprehensive risk assessment. J Magn Reson Imaging. 2017;46:338–53.
Mathur M, Jones JR, Weinreb JC. Gadolinium deposition and nephrogenic systemic fibrosis: a radiologist’s primer. Radiographics. 2020;40:153–62.
Martino F, Amici G, Rosner M, Ronco C, Novara G. Gadolinium-based contrast media nephrotoxicity in kidney impairment: the physio-pathological conditions for the perfect murder. J Clin Med. 2021;10:271.
Heshmatzadeh Behzadi A, Farooq Z, Newhouse JH, Prince MR. MRI and CT contrast media extravasation: a systematic review. Medicine (Baltimore). 2018;97: e0055.
Weinreb JC, Rodby RA, Yee J, Wang CL, Fine D, McDonald RJ, et al. Use of intravenous gadolinium-based contrast media in patients with kidney disease: consensus statements from the American college of radiology and the national kidney foundation. Kidney Med. 2021;3:142–50.
Schieda N, Blaichman JI, Costa AF, Glikstein R, Hurrell C, James M, et al. Gadolinium-based contrast agents in kidney disease: comprehensive review and clinical practice guideline issued by the Canadian association of radiologists. Can Assoc Radiol J. 2018;69:136–50.
Acknowledgements
The authors thank Nobuyuki Fukui for advice regarding the data management of this study and Mao Hayashi for editing a draft of this manuscript.
Funding
No funding was received for conducting this study.
Author information
Authors and Affiliations
Contributions
Conceptualization: JYI, RK; Data Curation: YK, TH, RW, HS; Formal analysis and investigation: YK, TH; Writing-original draft: YK, RW, HS; Writing-Review & Editing: JYI, RK; Supervision: RK.
Corresponding author
Ethics declarations
Conflict of interest
Takahiro Hirano and Ryozo Wakabayashi are employees of Clinical Study Support, Inc. Other authors declare that they have no conflict of interest.
Ethical approval
This retrospective, observational study was reviewed and approved by the Institutional Review Board of our hospital (IRB approval number: 20-196). This study was performed in line with the principles of the Declaration of Helsinki.
Informed consent
The requirement for signed informed consent was waived because of the retrospective nature of this study.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
This article is published under an open access license. Please check the 'Copyright Information' section either on this page or in the PDF for details of this license and what re-use is permitted. If your intended use exceeds what is permitted by the license or if you are unable to locate the licence and re-use information, please contact the Rights and Permissions team.
About this article
Cite this article
Kuwatsuru, Y., Hirano, T., Wakabayashi, R. et al. Changes in renal function over time in outpatients with eGFR ≥ 30 mL/min/1.73 m2: implication for timing of renal function testing before contrast-enhanced CT imaging. Jpn J Radiol 41, 994–1006 (2023). https://doi.org/10.1007/s11604-023-01425-y
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11604-023-01425-y