Abstract
Purpose
With advances in anti-diabetes drugs, increasing numbers of patients have high urinary glucose concentrations, which may alter magnetic resonance (MR) signal intensity. We sought to elucidate the effect of urinary glucose concentration and pH on transverse relaxation and MR signal intensity.
Materials and methods
The transverse relaxation rate (R2) was measured in samples with different glucose concentrations (in vitro) and in the urinary bladder of seven patients with diabetes and nine healthy volunteers (in vivo). The glucose concentration and pH in the in vitro samples and urine were measured. The signal intensity ratio of the bladder to adjacent tissues was obtained on T2-weighted imaging (WI), T1WI, and MR urography (in vivo). To clarify the effect of pH further, the urine of two healthy subjects was adjusted with acid and/or base to obtain various pH values (ex vivo).
Results
R2 increased significantly with high glucose concentrations in the in vitro study. In the in vivo study, high glucose concentration (p < 0.001) and low pH (p = 0.005) were significantly associated with high R2. R2 was higher (p = 0.002) and the signal in maximum-intensity projection images of MR urography was lower (p = 0.005) in patients with diabetes than in healthy subjects. Ex vivo study revealed that a decrease in pH in acid portion resulted in increased R2.
Conclusion
High concentrations of urinary glucose and low pH both enhance transverse relaxation, which, in turn, causes low signal intensity in urinary bladder on long echo time (TE) images, such as MR urography. Radiologists should be aware of this phenomenon when interpreting abnormally low-intensity bladders on long TE images.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
In general, fluid is hyperintense on T2-weighted images (T2WI) and hypointense on T1-weighted images (T1WI). Fluids that show intermediate to low signal intensity on T2WI are considered to contain protein or blood, such as T2WIs of paranasal sinusitis or ovarian tumors.
We encountered a patient with diabetes in whom urine in the bladder resulted in intermediate signal intensity on T2WI. The patient was receiving sodium‒glucose cotransporter 2 inhibitor treatment, and dipstick urinalysis was negative for occult blood in urine and urinary protein while strongly positive for urinary glucose.
Urinary glucose concentration is high in patients with diabetes and can be much higher in those treated with sodium-glucose cotransporter 2 inhibitors. The sodium-glucose cotransporter protein conveys nutrients into the cells. Sodium-glucose cotransporter 2, a type of sodium-glucose cotransporter found only in the kidney, reabsorbs 97% of glucose at the proximal convoluted tubules [1]. Accordingly, inhibition of sodium-glucose cotransporter 2 effectively lowers hyperglycemia by inhibiting glucose reabsorption in the kidneys, thus, increasing glucose excretion in the urine.
The glucose molecule has five unstable hydroxyl protons, with a weighted average chemical shift difference of 1.44 ppm at 22 °C [2]. Owing to chemical exchange between hydroxyl protons and the bulk water proton pool, glucose enhances transverse relaxation [2]. Yadav et al. reported the effects of glucose concentration and pH on the transverse relaxation rate (R2) in glucose solutions and in a in vivo mouse experiment [2]. However, the effect of these factors on signal intensity of urinary bladder, which is of clinical relevance, has not been investigated.
Intake of sodium-glucose cotransporter 2 inhibitors increases urinary glucose concentration and may result in low signal intensity of the urinary bladder on T2WI. Accordingly, this study aimed to elucidate the effect of urinary glucose concentration and pH on transverse relaxation and MR signal intensity.
Materials and methods
In vitro experiment
Sample preparation
A 50% glucose solution (Fuso Yakuhin Kogyo, Osaka, Japan) was diluted with physiological saline (Otsuka Pharmaceutical, Tokyo, Japan) to target concentrations of glucose solutions of 0, 50, 100, 200, 300, 500, and 700 mM. Then, the pH of the diluted glucose solutions was adjusted to pH 6 by adding dilutions of 0.1 mol/L hydrochloric acid (Kanto Chemical, Tokyo, Japan) and/or 0.01 mol/L sodium hydroxide (Kanto Chemical).
MRI examination and analysis
Each prepared solution was placed into a 7-mL tube (Insepack II-D, Tokuyama Sekisui, Shunan, Yamaguchi, Japan) and imaged using a 3-T clinical MR imaging (MRI) scanner (SIGNATMArchitect, v28, GE Healthcare, Milwaukee, WI, USA) with an AIR™ anterior array coil (GE Healthcare). The tubes were placed in a container filled with warm water to be maintained near body temperature. The tube temperature was 37 °C before scanning and 41 °C after scanning. T2 mapping was performed using the Carr–Purcell–Meiboom–Gill (CPMG) pulse sequence with the following parameters: repetition time (TR), 2000 ms; echo interval, 25 ms; number of acquired echoes, 12; echo time (TE), 25–300 ms (25 ms × 12); field-of-view (FOV), 200 × 200 mm; matrix, 128 × 160; slice thickness, 5 mm; number of slices, 1; bandwidth (BW), 15.6 kHz; and acquisition time, 5 min 24 s.
The images were then transferred to a workstation (Advantage Windows VolumeShare 7, GE Healthcare). Signal intensity was measured in a 60 mm2 region-of-interest (ROI) on the images of each tube. R2 was calculated as the slope between the logarithm of the signal intensity and TE using the least-squares method (Microsoft Excel, Microsoft, Redmond, WA, USA).
Glucose concentration (JCA-ZS050™, JEOL, Tokyo, Japan) and pH (PICCORO + ™, Hanna Instruments Japan, Chiba, Japan) were measured after MRI examination.
In vivo experiment
Subjects
This study was approved by the ethics committee of our institution (19,521). After receiving an explanation of the procedure, seven patients taking sodium-glucose cotransporter 2 inhibitors (two men and five women; age range 39–72 years) and nine healthy subjects (nine men; age range 22–24 years) provided written consent for participation in the study. The experiment period was from July 16, 2020 to December 16, 2021.
MR examinations
All MR examinations were performed during the time period of 12:00–13:00 using the same MRI equipment and coil as in in vitro scans, with the following sequences: T2WI (fast recovery fast spin echo; TR 4100 ms; TE 82.3 ms; FOV 240 × 240 mm; matrix, 400 × 320; slice thickness, 5 mm; BW 62.5 kHz), T2 mapping (TR 2000 ms; echo interval, 25 ms; number of acquired echoes, 12; TE 25–300 ms; FOV 240 × 240 mm; matrix, 128 × 160; slice thickness, 5 mm; number of slices, 1; BW 15.6 kHz), T1WI (liver acquisition with volume acceleration; TR 8.5 ms; TE 2.7 ms; FOV 280 × 280 mm; matrix, 256 × 160; slice thickness, 5 mm; BW 142.9 kHz), and MR urography (3D breath hold fast recovery fast spin echo; TR 1800 ms; TE 630 ms; FOV 360 × 324 mm; matrix, 256 × 160 mm; slice thickness, 3.6 mm; BW 83.33 kHz). In addition, apparent diffusion coefficient was measured on diffusion-weighted imaging (b = 0, 1000 s/mm2; TR 7500 ms; TE 68.8 ms; FOV 240 × 240 mm; matrix, 128 × 128; slice thickness, 10 mm; BW 250 kHz) acquired in five patients and in all healthy subjects.
Urinalysis
The pH (PICCORO + ™, Hanna Instruments Japan) as well as the protein and glucose concentrations (JCA-ZS050™, JEOL) were measured in urine collected immediately after MRI examination. The number of red blood cells per high-power field was counted in five patients and in all healthy subjects. Urinary blood concentration was measured in four patients and in eight healthy subjects (JCA-ZS050™, JEOL). Although it was confirmed that it was not hematuria with the naked eye, additional measurements were taken to determine more quantitatively whether it was hematuria.
Image analysis
To determine the optimal region in the urinary bladder for ROI placement, the means and standard deviations of signal intensities in six ROIs were measured on T2WI in four individuals. These ROIs were located at right ventral, center ventral, left ventral, right dorsal, center dorsal, and left ventral region in the urinary bladder. Comparison of coefficient of variation between the ventral three ROIs and dorsal three ROIs revealed that it was significantly larger in the ventral group, indicating that the signal heterogeneity was larger in the ventral portion of the bladder. Among three ROIs in the dorsal portion, based on the fact that the ureteric jet can be observed at center dorsal region in clinical practice, all bladder ROIs were placed in the right dorsal corner.
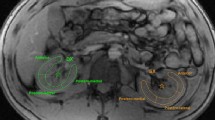
R2 was calculated in the same manner as in the in vitro experiment (ROI area 94–105 mm2). The apparent diffusion coefficient of urine in the bladder was calculated from the images acquired at b = 0 and b = 1000 s/mm2 (ROI area 96–105 mm2). To evaluate the signal intensity of the bladder, a ROI was placed in the bladder (ROI area 98–105 mm2) and iliopsoas (98–104 mm2) on T1WI, in the bladder (98–101 mm2) and pararectal fat (98–101 mm2) on T2WI, and in the bladder (495–508 mm2) and cerebrospinal fluid in the thecal sac at the L3–L4 level (100–108 mm2) on a maximum-intensity projection (MIP) image of MR urography (Fig. 1). Contrast ratios were calculated as (signal intensity in the bladder)/(signal intensity of iliopsoas, fat, or thecal sac). The above measurements were performed by one board-certified radiologist and one radiologic technologist independently.
Ex vivo experiment
Sample preparation
To investigate the effect of pH on MRI signals further, the pH of the urine of two healthy subjects (ex vivo #1 and #2) was adjusted to pH 4.5–7.5 by adding hydrochloric acid and/or sodium hydroxide as in the in vitro study. Strong acids and bases were used in this process to minimize the dilution of urinary components. MRI examination was performed as in the in vitro study.
Statistical analysis
Statistical analyses were performed using IBM SPSS Statistics version 27.0 (IBM Corp., Armonk, NY, USA). Spearman’s rank correlation was used to evaluate the effects of glucose concentration on R2 in vitro study. The intraclass correlation coefficients (ICCs) were used to evaluate inter-rater agreement. The agreement was classified as poor (ICC < 0.40), fair (ICC = 0.40–0.59), good (ICC = 0.60–0.75), or excellent (ICC > 0.75). Unless poor agreement was observed, the mean value of these two measurements was used in further analysis. In the in vivo study, Spearman’s rank correlation was used to evaluate the effects of glucose concentration and pH on R2. Moreover, the Mann–Whitney U test was used to compare R2 and the signal intensity ratios between patients and healthy subjects. Statistical significance was set at p < 0.05.
Results
In vitro experiment
Figure 2 shows representative T2WI of the in vitro simulated samples of urine. Signals decreased with increasing glucose concentration and longer TE. Figure 3 shows the relationship between glucose concentration and R2 in the in vitro and in vivo measurements. R2 increased with increasing glucose concentration in vitro. Spearman’s rank correlation revealed significant positive correlation (p < 0.001) in the in vitro experiment.
T2 mapping of samples of different glucose concentrations. Representative T2 mapping images were acquired with an echo time (TE) of 25, 100, and 200 ms for various glucose concentrations (I–VII). (VIII) Additional plastic tubes of 5% glucose solution (277.78 mM) (Otsuka Pharmaceutical, Tokyo, Japan) were added to stabilize the sample tubes
Relationship between R2 and glucose concentration in all samples. The relationship between glucose concentration and R2 was assessed both in vitro and in vivo. R2 increased as glucose concentration increased. The trend was similar in in vitro samples and in the patient’s urine. In the in vivo study, Spearman’s rank correlation revealed significant positive correlation between glucose concentration and R2 (p < 0.001). Each in vivo symbol represents the urinalysis results of one patient or a healthy subject. ●, in vitro sample; ○, urine of patients; △, urine of healthy subjects
In vivo experiment
ICCs revealed good agreement between Rater 1 versus Rater 2 (0.99 in R2, 0.92 in apparent diffusion coefficient, 0.72 in T1WI, 0.85 in T2WI, 0.99 in MR urography). Based on these analyses, the mean value of two raters was used for further analysis.
Spearman’s rank correlation of all participant (seven patients plus nine healthy subjects) revealed significant positive correlation between glucose concentration and R2 (p < 0.001) (Fig. 3) and also revealed significant negative correlation between pH and R2 (p = 0.005) (Fig. 4).
Relationship between R2 and pH in in vivo and ex vivo experiments. The 〇 indicates patients (in vivo) and △ indicates healthy subjects (in vivo). In the in vivo study, Spearman’s rank correlation revealed a significant negative correlation between pH and R2 (p = 0.005). The ▲ indicates ex vivo #1, and the ▼ indicates ex vivo #2. pH adjustment of samples using only HCl and NaOH revealed that decreasing pH in acid portion increased R2 (ex vivo data)
Urinalysis showed the highest protein concentration was 57.37 mg/dL. It also showed that the highest blood volume was 454 ng/mL. The number of red blood cells of this case was 5–10 per high-power field.
R2 (Fig. 5a) and contrast ratios on MR urography (Fig. 5b), T1WI (Fig. 5c), and T2WI (Fig. 5d) were compared between the patients and healthy subjects using the Mann–Whitney U test. Of note, R2 was significantly higher in the patients than in healthy subjects (p = 0.002). In addition, the MR urography contrast ratio was significantly lower in the patients than in healthy subjects (p = 0.005). However, there was no significant difference in the other imaging metrics, such as the apparent diffusion coefficient, between the patients and healthy subjects (T1WI, p = 0.92; T2WI, p = 0.71; apparent diffusion coefficient, p = 0.70).
Comparison of transverse relaxation rate (R2) and contrast ratio on magnetic resonance urography (MRU) and T1-(T1WI) and T2-weighted images (T2WI) between patients and healthy subjects. a Transverse relaxation rate, R2, b contrast ratio in MRU, c contrast ratio in T1WI, d contrast ratio in T2WI. Upper end, inside bar, and lower end of box signifies 75, 50, 25 percentiles. End points of vertical line signify the maximum and minimum value of measurement. Both R2 and the contrast ratio of MRU were significantly different between patients and healthy subjects. *Statistically significant. In b, the data of the healthy subjects show a large variation than the patients. This is thought to be attributed to the curvature of exponential. Since signal intensity on T2WI is proportional to exp (-R2 TE) (TE: echo time) and the TE in MRU is long, signal intensity change for unit change of R2 is large when R2 is nearly zero as compared to that of a larger R2. As R2 of healthy subjects is smaller than that in patients, the variation in healthy subjects is larger
Figure 6 shows representative T1WIs and T2WIs and the MIP of MR urography obtained in a patient and a healthy subject.
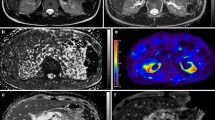
Magnetic resonance images obtained in a patient and in a healthy subject. a T1-weighted imaging (T1WI), b T2-weighted imaging (T2WI), c maximum-intensity projection images of magnetic resonance (MR) urography. Upper row: images of a patient receiving sodium-glucose cotransporter 2 inhibitor treatment (urinary glucose concentration, 532.27 mM); lower row: images of a healthy subject (urinary glucose concentration, 0.05 mM). The urinary bladder and other urinary tracts could be visualized on MR urography in the healthy subject but not in the patient (arrows on 1-c and 2-c). The signal intensity of the urinary bladder on T1- and T2WI appears normal at first glance; however, when compared with the signal of fluid in the hip joint (arrows on 1-b and 2-b), that in the bladder of the patient is somewhat lower
Ex vivo experiment
The results of the ex vivo study are presented in Fig. 4. Lowering pH by adding small amount of hydrochloric acid and/or sodium hydroxide was performed in three steps for each subject. Among six steps, one step, ex vivo #1 from pH 7.55 to 6.50, traversed neutral line (pH7) and R2 slightly decreased. The other five steps in acid portion were associated with increase in R2.
Discussion
Glucose concentration and pH affect urinary R 2 and signal intensity on T2WI
In this study, we showed that high urinary glucose concentration and low urinary pH increased the R2 value and could cause a signal loss on heavily T2WI. Even in conventional T2WI, a low signal might occur when the TE is set to 100 ms at a urinary glucose concentration of approximately 460 mM (approximately 10%; Fig. 2). Notably, low signal intensity in the bladder on T2WIs does not necessarily have pathological significance.
Mechanisms by which glucose enhances transverse relaxation
In the present in vitro study, R2 increased as glucose concentration increased to 650 mM. This is in concordance with the findings of previous report that increase of glucose concentration from 0 to 20 mM is associated with linear increase of R2 [2]. Generally, the transverse relaxation of water protons in an aqueous solution containing carbohydrates and proteins is enhanced compared with that in pure water because of the exchange of protons between different states of chemical shift [3]. Chemical shift of hydroxyl protons of glucose at − 14 °C ranges in 6.03–8.07 ppm [4]. A weighted average chemical shift difference between the protons of the hydroxyl group of glucose and the protons of water is 1.44 ppm at 22 °C [2]. Therefore, chemical exchange leads to a phase difference among the protons, which enhances transverse relaxation, thereby reducing the signal intensity [2].
The effect on transverse relaxation per unit concentration of solute (transverse relaxivity), r2ex (s−1 mM−1), can be obtained from the following equation [2]:
where kex is the chemical exchange rate, PB is the number of exchangeable solute protons for 110 M proton atoms in 1 L of water, and Δω is the chemical shift difference. Transverse relaxivity peaks when kex = Δω. Glucose has the following parameters: kex = 4600 s−1 (at 37 °C), PB = 5 mM, and Δω = 1.44 ppm (at 22 °C) [2]. Glucose has been reported to have a high transverse relaxation capacity because it has a value close to kex = Δω (Fig. 7) [2].
Relationship between chemical exchanges (kex) and transverse relaxivity (r2ex) of glucose and urea. The relationship between r2ex and kex was obtained from Eq. (1). In this calculation, Δω was set to be 1.44 ppm for glucose [2] and 1.0 ppm for urea [5]. The black line represents glucose, the dotted line represents urea, and ○ and △ represent the kex and r2ex of glucose and urea at pH 7.3 [2] and pH 7.4 [5] at 37 °C, respectively. Glucose has a value close to kex = Δω (the maximum) and, thus, has a high transverse relaxivity. In contrast, urea has a value far from the maximum and, thus, has a low transverse relaxivity
In addition to glucose, urine contains various other components, such as urea, creatine, ammonia, and uric acid. Urea, which has the highest concentration and is thought to be related to transverse relaxation, has four exchangeable protons, a chemical exchange rate of kex = 4.60 (1/s), and Δω = 1.0 ppm at pH 7.37 and 37 °C [5]. Figure 7 shows that urea does not satisfy kex = Δω; therefore, its transverse relaxivity is small. In the quantitative analysis of Eq. (1), the relaxivity of urea, r2ex(urea), was 1.1 × 10–2 times that of glucose, r2ex(glc); thus, it was not involved in the signal reduction of urine.
Similarly, the transverse relaxivity of eight renal metabolites (alanine, choline, citric acid, creatine, lactate, lysine, myoinositol, and taurine) was determined using Eq. (1). The transverse relaxation rate (s−1) was calculated by multiplying the transverse relaxivity (s−1 mM−1) by the concentration (mM). The rates were low, mainly because of their low kex and low urine concentrations. Indeed, the transverse relaxivity and concentration of myoinositol, which has the highest relaxivity among the eight renal metabolites, are approximately 0.6 times and 0.6 times those of glucose in normal urine, respectively [2, 5].
Thus, in the urine, only glucose has a kex almost equal to Δω. The fact that the kex of glucose is higher than that of other carbohydrates is responsible for its enhanced transverse relaxation.
Effect of pH on transverse relaxation
In this study, R2 of healthy subjects varied from 0.76 to 2.69, even though their urine samples contained little glucose. The in vivo results of urine from each subject showed that low pH was significantly associated with high R2.
The rate of chemical exchange can be expressed by the following equation [3]:
where kex is the rate constant of the chemical exchange of the solution and Kw is the solubility product of water. When a solution is acidic, the first term on the right side becomes dominant, and when it is alkaline, the second term on the right side becomes dominant. The third term remains nearly constant under physiological conditions. According to this equation, an acidic pH increases the chemical exchange rate kex and eventually the transverse relaxivity of the sample in most solutes. The mechanism that causes R2 to increase as the urine becomes more acidic can be explained as shown in Fig. 7. Many renal metabolites (except glucose), such as urea, have kex much lower than Δω, as shown in Fig. 7 [5]. As kex increases, the transverse relaxivity of urea moves to the left side of the graph in Fig. 7. Similar to urea, the transverse relaxivity of other renal metabolites is expected to increase with decreasing pH. Although the effect of individual solutes is small, the summation of the individual solutes increases the overall relaxation rate of the urine due to decreased pH.
The results of this study suggest that urinary pH is a factor that determines the signal intensity on T2WI. The urine becomes acidic due to strenuous exertion or diet [6, 7]. Generally, meat-containing meals make the urine acidic, while a diet of fruits and vegetables makes it alkaline [6, 7]. To obtain good MR urography, it is preferable to avoid strenuous exertion and meat before examination.
Other factors that may cause low signal on T2WIs
A low signal in urine on T2WIs could be mistaken for pathological findings, such as high protein levels or hemorrhage.
Protein
The transverse relaxivity of bovine serum albumin is 7.6 mM−1 s−1 at 14.1 T [8] and 6.3 mM−1 s−1 at 3 T (our unpublished data). Under an assumption that all proteins are albumin (molecular weight 66,000 Da), the healthy value of protein in the urine (0.1 g/L) [9] would correspond to 1.5 × 10–3 mM of albumin concentration; if the transverse relaxivity is 7 mM−1 s−1, R2 would be 1.1 × 10–2 s−1, indicating a negligible effect on the transverse relaxation. In another estimation with the same assumptions, R2 of 1.0 s−1 corresponds to 8.58 g/L of protein, indicating a nephrotic state. None of the patients or healthy subjects in this study had such a high urinary protein level.
Hemorrhage
R2 of blood at 3 T can be obtained using blood oxygen saturation (Y), hematocrit value (Hct), and the CPMG echo interval (τCPMG) [10]. The partial pressure of oxygen in urine is approximately 40 mmHg [11], which corresponds to approximately Y = 0.8 according to the hemoglobin saturation curve [12]. With the parameters of Y = 0.8, Hct = 0.4, and τCPMG = 20 ms, R2 is reported to be T2 = 100 ms (R2 = 10 s−1) [13]. Assuming that blood is diluted by one-tenth in hematuria and the R2 value is also 1/10, the equivalent R2 of the hematuria is 1.0 s−1. This corresponds to gross hematuria because 0.1% blood is sufficient to identify hematuria with the naked eye [14]. None of the patients or healthy subjects in this study had a high amount of blood in their urine.
Viscosity of urine
There was no significant difference in the apparent diffusion coefficient among five patients and nine healthy subjects, suggesting that R2 was not affected by viscosity in this study.
Limitations
A limitation of our study was the small number of subjects. During the COVID-19 pandemic, it was difficult to recruit patients with diabetes. Nevertheless, a clear tendency was revealed in this study. Since the number of subjects in this study was small, it is thought that better results would be obtained if the experiment is conducted with a larger number of subjects.
Under ideal conditions, control subjects should be age- and sex-matched to the patients. Healthy ranges of urinary ingredients do not change according to age or sex, and factors other than glucose concentration and pH within the healthy range do not affect transverse relaxation; thus, using only young men as the healthy controls might have no adverse effect on the results.
Conclusions
In patients with diabetes taking sodium-glucose transporter 2 inhibitors, high urinary glucose concentrations can lead to decreased signals in the bladder and urinary tract on T2WIs with long TE, such as MR urography. Low pH can also decrease the signal intensity on T2WI. Therefore, while interpreting the low signal on T2WI, the history of sodium-glucose transporter 2 inhibitor use should be checked before diagnosing proteinuria or hematuria. To obtain good MR urography, measures should be taken to avoid strenuous activity or intake of certain foods.
Availability of data and materials
The datasets generated during and/or analyzed during the current study are available from the corresponding author on reasonable request.
Code availability
Not applicable.
References
Nespoux J, Vallon V. SGLT2 inhibition and kidney protection. Clin Sci (Lond). 2018;132:1329–39. https://doi.org/10.1042/CS20171298.
Yadav NN, Xu J, Bar-Shir A, Qin Q, Chan KW, Grgac K, et al. Natural D-glucose as a biodegradable MRI relaxation agent. Magn Reson Med. 2014;72:823–8. https://doi.org/10.1002/mrm.25329.
Fabri D, Williams MA, Halstead TK. Water T2 relaxation in sugar solutions. Carbohydr Res. 2005;340:889–905. https://doi.org/10.1016/j.carres.2005.01.034.
Brown GD, Bauer J, Osborn HMI, Kuemmerle R. A solution NMR approach to determine the chemical structures of carbohydrates using the hydroxyl groups as starting points. ACS Omega. 2018;3:17957–75. https://doi.org/10.1021/acsomega.8b02136.
Stabinska J, Neudecker P, Ljimani A, Wittsack HJ, Lanzman RS, Müller-Lutz A. Proton exchange in aqueous urea solutions measured by water-exchange (WEX) NMR spectroscopy and chemical exchange saturation transfer (CEST) imaging in vitro. Magn Reson Med. 2019;82:935–47. https://doi.org/10.1002/mrm.27778.
Welch AA, Mulligan A, Bingham SA, Khaw KT. Urine pH is an indicator of dietary acid–base load, fruit and vegetables and meat intakes: results from the European Prospective Investigation into Cancer and Nutrition (EPIC)-Norfolk population study. Br J Nutr. 2008;99:1335–43. https://doi.org/10.1017/S0007114507862350.
Barrett KE, Barman SM, Boitano S, Brooks HL. Ganong’s review of medical physiology. 25th ed. New York: McGraw-Hill Education; 2016. p. 842–3.
Daoust A, Dodd S, Nair G, Bouraoud N, Jacobson S, Walbridge S, et al. Transverse relaxation of cerebrospinal fluid depends on glucose concentration. Magn Reson Imaging. 2017;44:72–81. https://doi.org/10.1016/j.mri.2017.08.001.
Kolbach-Mandel AM, Mandel NS, Hoffmann BR, Kleinman JG, Wesson JA. Stone former urine proteome demonstrates a cationic shift in protein. Urolithiasis. 2017;45:337–46. https://doi.org/10.1007/s00240-017-0969-y.
Chen JJ, Pike GB. Human whole blood T2 relaxometry at 3 Tesla. Magn Reson Med. 2009;61:249–54. https://doi.org/10.1002/mrm.21858.
Leonhardt KO, Landes RR. Oxygen tension of the urine and renal structures. Preliminary report of clinical findings. N Engl J Med. 1963;269:115–21. https://doi.org/10.1056/NEJM196307182690301.
Edward JH. Guyton and Hall textbook of medical physiology, 13th edn. Philadelphia, PA: Elsevier; 2016. p. 530.
Lu H, Xu F, Grgac K, Liu P, Qin Q, van Zijl P. Calibration and validation of TRUST MRI for the estimation of cerebral blood oxygenation. Magn Reson Med. 2012;67:42–9. https://doi.org/10.1002/mrm.22970.
Wong LM, Chum JM, Maddy P, Chan STF, Travis D, Lawrentschuk N. Creation and validation of a visual macroscopic hematuria scale for optimal communication and an objective hematuria index. J Urol. 2010;184:231–6. https://doi.org/10.1016/j.juro.2010.03.018.
Acknowledgements
We are grateful to Drs. Kazuki Fukuchi and Izumi Imaoka for their inspiration.
Funding
No funding was received.
Author information
Authors and Affiliations
Contributions
Guarantors of integrity of the entire study, all the authors; study concept/study design, all authors; data acquisition; SY, SK, and MH; analysis and interpretation of data, SY, HT, JK, HT, and YH; manuscript drafting or manuscript revision for important intellectual content, all the authors; approval of the final version of the submitted manuscript, all the authors; agreement to be accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work, all the authors.
Corresponding author
Ethics declarations
Conflict of interest
All the authors disclose no relevant conflicts of interest.
Ethics approval
This study was approved by the Ethics Committee of our institution (19521). All the procedures performed in this study were in accordance with the 1964 Helsinki Declaration and its later amendments or comparable ethical standards.
Consent to participate
Written informed consent was provided by all participants.
Consent for publication
Written informed consent was provided by all participants.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
About this article
Cite this article
Yoshimura, S., Tanaka, H., Kawabata, S. et al. Effect of urinary glucose concentration and pH on signal intensity in magnetic resonance images. Jpn J Radiol 40, 930–938 (2022). https://doi.org/10.1007/s11604-022-01273-2
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11604-022-01273-2