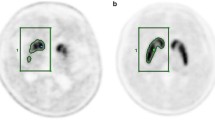
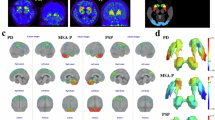
Abstract
Purpose
To investigate the correlation between the striatal three-dimensional location and the Unified Parkinson’s Disease Rating Scale (UPDRS) motor score in the context of idiopathic Parkinson’s disease (PD) through radiolabeled N-(3-fluoropropyl)-2β-carboxymethoxy-3β-(4-iodophenyl) nortropane positron emission tomography/computed tomography (FP-CIT PET/CT).
Materials and methods
In this cross-sectional study, we assessed the UPDRS motor score and performed FP-CIT PET/CT in patients with PD. Thirty-eight patients with idiopathic PD [average 70 years of age (range 49–86); male:female ratio 12:26] were enrolled. The correlation between FP-CIT PET/CT and the UPDRS III scores was investigated after the transformation of PET images by an alternative method using MATLAB.
Results
Left caudate nucleus uptake negatively correlated with UPDRS items 18, 20 (face), 22 (right arm and leg), 23, 24 (right side), 26 (right side), 27, 30, and 31, while right caudate nucleus uptake positively correlated with items 18, 22 (left arm), 26, and 29. Putamen uptake correlated with items 22 and 25. Left caudate nucleus uptake positively correlated with axial and akinetic-rigidity symptoms.
Conclusions
FP-CIT uptake in specific basal ganglia structures strongly correlated with the UPDRS III motor score. Among these, the left caudate nucleus exhibited the strongest relationship with axial and akinetic-rigidity PD symptoms.




Similar content being viewed by others
References
Djaldetti R, Treves TA, Ziv I, Melamed E, et al. Use of a single [(123)I]-FP-CIT SPECT to predict the severity of clinical symptoms of Parkinson disease. Neurol Sci. 2009;30(4):301–5.
Eggers C, Kahraman D, Fink GR, et al. Akinetic-rigid and tremor-dominant Parkinson’s disease patients show different patterns of FP-CIT single photon emission computed tomography. Mov Disord. 2011;26:416–23.
Jellinger KA. Post mortem studies in Parkinson’s disease—is it possible to detect brain areas for specific symptoms? J Neural Transm Suppl. 1999;56:1–29.
Paulus W, Jellinger K. The neuropathologic basis of different clinical subgroups of Parkinson’s disease. J Neuropathol Exp Neurol. 1991;50:743–55.
Rajput AH, Voll A, Rajput ML, Robinson CA, et al. Course in Parkinson disease subtypes: a 39-year clinicopathologic study. Neurology. 2009;73:206–12.
Zaidel A, Arkadir D, Israel Z, et al. Akineto-rigid vs. tremor syndromes in Parkinsonism. Curr Opin Neurol. 2009;22:387–93.
Helmich RC, Hallett M, Deuschl G, Toni I, et al. Cerebral causes and consequences of parkinsonian resting tremor: a tale of two circuits? Brain. 2012;135:3206–26.
Hilker R, Schweitzer K, Coburger S, et al. Nonlinear progression of Parkinson disease as determined by serial positron emission tomographic imaging of striatal fluorodopa F 18 activity. Arch Neurol. 2005;62:378–82.
Jankovic J, Kapadia AS. Functional decline in Parkinson disease. Arch Neurol. 2001;58:1611–5.
Louis ED, Tang MX, Cote L, et al. Progression of parkinsonian signs in Parkinson disease. Arch Neurol. 1999;56:334–7.
Zetusky WJ, Jankovic J, Pirozzolo FJ. The heterogeneity of Parkinson’s disease: clinical and prognostic implications. Neurology. 1985;35:522–6.
Spiegel J, Hellwig D, Samnick S, et al. Striatal FP-CIT uptake differs in the subtypes of early Parkinson’s disease. J Neural Transm. 2007;114:331–5.
Stoessl AJ. Radionuclide scanning to diagnose Parkinson disease: is it cost-effective? Nat Clin Pract Neurol. 2009;5:10–1.
de Lau LM, Breteler MM. Epidemiology of Parkinson’s disease. Lancet Neurol. 2006;5:525–35.
Hubbuch M, Farmakis G, Schaefer A, et al. FP-CIT SPECT does not predict the progression of motor symptoms in Parkinson’s disease. Eur Neurol. 2011;65:187–92.
Ottaviani S, Tinazzi M, Pasquin I, et al. Comparative analysis of visual and semi-quantitative assessment of striatal [123I] FP-CIT-SPET binding in Parkinson’s disease. Neurol Sci. 2006;27:397–401.
Kahraman D, Eggers C, Schicha H, et al. Visual assessment of dopaminergic degeneration pattern in 123I-FP-CIT SPECT differentiates patients with atypical parkinsonian syndromes and idiopathic Parkinson’s disease. J Neurol. 2012;259:251–60.
O’Brien JT, Colloby S, Fenwick J, et al. Dopamine transporter loss visualized with FP-CIT SPECT in the differential diagnosis of dementia with Lewy bodies. Arch Neurol. 2004;61:919–25.
Papathanasiou N, Rondogianni P, Chroni P, Datseris I, et al. Interobserver variability, and visual and quantitative parameters of 123I-FP-CIT SPECT (DaTSCAN) studies. Ann Nucl Med. 2012;26:234–40.
Vingerhoets FJ, Schulzer M, Calne DB, et al. Which clinical sign of Parkinson’s disease best reflects the nigrostriatal lesion? Ann Neurol. 1997;41:58–64.
Wang J, Zuo CT, Jiang YP, et al. 18F-FP-CIT PET imaging and SPM analysis of dopamine transporters in Parkinson’s disease in various Hoehn & Yahr stages. J Neurol. 2007;254:185–90.
Kazumata K, Dhawan V, Chaly T, et al. Dopamine transporter imaging with fluorine-18-FPCIT and PET. J Nucl Med. 1998;39:1521–30.
Benamer HT, Patterson J, Wyper DJ, et al. Correlation of Parkinson’s disease severity and duration with 123I-FP-CIT SPECT striatal uptake. Mov Disord. 2000;15:692–8.
Berti V, Pupi A, Ramat S, et al. Clinical correlation of the binding potential with 123I-FP-CIT in de novo idiopathic Parkinson’s disease patients. Eur J Nucl Med Mol Imaging. 2008;35:2220–6.
Ma Y, Dhawan V, Mentis M, et al. Parametric mapping of [18F]FPCIT binding in early stage Parkinson’s disease: a PET study. Synapse. 2002;45:125–33.
Acknowledgments
This work was supported by the National Research Foundation of Korea Grant funded by the Korean Government (NRF-2012R1A1A1042282).
Conflict of interest
The authors declare that they have no competing interests.
Author information
Authors and Affiliations
Corresponding author
About this article
Cite this article
Chung, M., Park, Y.S., Kim, J.S. et al. Correlating Parkinson’s disease motor symptoms with three-dimensional [18F]FP-CIT PET. Jpn J Radiol 33, 609–618 (2015). https://doi.org/10.1007/s11604-015-0427-0
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11604-015-0427-0