Abstract
Objective
Previous studies have shown that the autonomic nervous system (ANS), which can be affected by emotions, is important in the occurrence or progression of glaucoma. The autonomic innervation distributed in the anterior chamber (AC) structures might play an efferent role in the neural regulation of intraocular pressure (IOP). This study aimed to investigate the anatomic neural connection from the emotional brain to autonomic innervation in the AC.
Methods
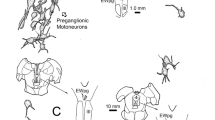
A retrograde trans-multisynaptic pseudorabies virus encoded with an enhanced green fluorescent protein (PRV531) and non-trans-synaptic tracer FAST Dil were injected into the right eye of mice, respectively. Fluorescent localization in the emotional brain and preganglionic nuclei was studied. Five and a half days after PRV531 injection into the right AC, fluorescent signals were observed in several emotional brain regions, including the amygdala, agranular insular cortex, lateral septal nuclei, periaqueductal gray, and hypothalamus. Autonomic preganglionic nuclei, including Edinger-Westphal nucleus, superior salivatory nucleus, and intermediolateral nucleus, were labeled using PRV531.
Results
The sensory trigeminal nuclei were not labeled using PRV531. The fluorescence signals in the nuclei mentioned above showed bilateral distribution, primarily on the ipsilateral side. Seven days after injecting FAST Dil into the AC, we observed no FAST Dil-labeled neurons in the central nervous system.
Conclusion
Our results indicate a neural connection from the emotional brain to autonomic innervation in the AC, which provides anatomical support for the emotional influence of IOP via the ANS.
Similar content being viewed by others
References
Mabuchi F, Yoshimura K, Kashiwagi K, et al. High prevalence of anxiety and depression in patients with primary open-angle glaucoma. J Glaucoma, 2008,17(7):552–557
Kong X, Yan M, Sun X, et al. Anxiety and Depression are More Prevalent in Primary Angle Closure Glaucoma Than in Primary Open-Angle Glaucoma. J Glaucoma, 2015,24(5):57–63
Zhou C, Qian S, Wu P, et al. Anxiety and depression in Chinese patients with glaucoma: sociodemographic, clinical, and self-reported correlates. J Psychosom Res, 2013,75(1):75–82
Zhang X, Liu Y, Wang W, et al. Why does acute primary angle closure happen? Potential risk factors for acute primary angle closure. Surv Ophthalmol, 2017,62(5):635–647
Lin HC, Chien CW, Hu CC, et al. Comparison of comorbid conditions between open-angle glaucoma patients and a control cohort: a case-control study. Ophthalmology, 2010,117(11):2088–2095
Skalicky S, Goldberg I. Depression and quality of life in patients with glaucoma: a cross-sectional analysis using the Geriatric Depression Scale-15, assessment of function related to vision, and the Glaucoma Quality of Life-15. J Glaucoma, 2008,17(7):546–551
Lim NC, Fan CH, Yong MK, et al. Assessment of Depression, Anxiety, and Quality of Life in Singaporean Patients With Glaucoma. J Glaucoma, 2016,25(7):605–612
Zhang X, Olson DJ, Le P, et al. The Association Between Glaucoma, Anxiety, and Depression in a Large Population. Am J Ophthalmol, 2017,183:37–41
Berchuck S, Jammal A, Mukherjee S, et al. Impact of anxiety and depression on progression to glaucoma among glaucoma suspects. Br J Ophthalmol, 2020, 105(9):1244–1249
Jung Y, Han K, Wang SM, et al. Effect of depressive symptom and depressive disorder on glaucoma incidence in elderly. Sci Rep, 2021,11(1):5888
Shin DY, Jung KI, Park HYL, et al. The effect of anxiety and depression on progression of glaucoma. Sci Rep, 2021,11(1):1769
Zhou RX, Li F, Gao K, et al. Effects of different types of music on intraocular pressure and the underlying mechanism. Zhonghua Yan Ke Za Zhi (Chinese), 2020,56(1):25–31
Kaluza G, Strempel I. Effects of self-relaxation methods and visual imagery on IOP in patients with open-angle glaucoma. Ophthalmologica, 1995,209(3):122–128
Jasien JV, Girkin CA, Downs JC. Effect of Anesthesia on Intraocular Pressure Measured With Continuous Wireless Telemetry in Nonhuman Primates. Invest Ophthalmol Vis Sci, 2019,60(12):3830–3834
Keil MF, Briassoulis G, Gokarn N, et al. Anxiety phenotype in mice that overexpress protein kinase A. Psychoneuroendocrinology, 2012,37(6):836–843
Kreibig SD. Autonomic nervous system activity in emotion: a review. Biol Psychol, 2010,84(3):394–421
Volders PG. Novel insights into the role of the sympathetic nervous system in cardiac arrhythmogenesis. Heart Rhythm, 2010,7(12):1900–1906
Fournier A, Mondillon L, Luminet O, et al. Interoceptive Abilities in Inflammatory Bowel Diseases and Irritable Bowel Syndrome. Front Psychiatry, 2020,11:229
Pellissier S, Bonaz B. The Place of Stress and Emotions in the Irritable Bowel Syndrome. Vitam Horm, 2017, 103:327–354
Fukudo S. Stress and visceral pain: focusing on irritable bowel syndrome. Pain, 2013,154(Suppl 1):S63–S70
Wehrwein EA, Orer HS, Barman SM. Overview of the Anatomy, Physiology, and Pharmacology of the Autonomic Nervous System. Compr Physiol, 2016,6(3):1239–1278
Carnevali L, Montano N, Statello R, et al. Rodent models of depression-cardiovascular comorbidity: Bridging the known to the new. Neurosci Biobehav Rev, 2017,76(Pt A):144–153
Bajkó Z, Szekeres CC, Kovács KR, et al. Anxiety, depression and autonomic nervous system dysfunction in hypertension. J Neurol Sci, 2012,317(1–2):112–116
Sgoifo A, Carnevali L, Alfonso Mde L, et al. Autonomic dysfunction and heart rate variability in depression. Stress, 2015,18(3):343–352
Budavari AI, Olden KW. Psychosocial aspects of functional gastrointestinal disorders. Gastroenterol Clin North Am, 2003, 32(2):477–506
Fond G, Loundou A, Hamdani N, et al. Anxiety and depression comorbidities in irritable bowel syndrome (IBS): a systematic review and meta-analysis. Eur Arch Psychiatry Clin Neurosci, 2014,264(8):651–660
Jordan C, Sin J, Fear NT, et al. A systematic review of the psychological correlates of adjustment outcomes in adults with inflammatory bowel disease. Clin Psychol Rev, 2016,47:28–40
Furness JB, Callaghan BP, Rivera LR, et al. The enteric nervous system and gastrointestinal innervation: integrated local and central control. Adv Exp Med Biol, 2014,817:39–71
Salvioli B, Pellegatta G, Malacarne M, et al. Autonomic nervous system dysregulation in irritable bowel syndrome. Neurogastroenterol Motil, 2015,27(3):423–430
Myers B, Greenwood-Van Meerveld B. Role of anxiety in the pathophysiology of irritable bowel syndrome: importance of the amygdala. Front Neurosci, 2009,3:47
Liu Q, Wang EM, Yan XJ, et al. Autonomic functioning in irritable bowel syndrome measured by heart rate variability: a meta-analysis. J Dig Dis, 2013,14(12):638–646
Sadowski A, Dunlap C, Lacombe A, et al. Alterations in Heart Rate Variability Associated With Irritable Bowel Syndrome or Inflammatory Bowel Disease: A Systematic Review and Meta-Analysis. Clin Transl Gastroenterol, 2020,12(1):e00275
La Rovere MT, Christensen JH. The autonomic nervous system and cardiovascular disease: role of n-3 PUFAs. Vascul Pharmacol, 2015,71:1–10
Eckberg DL, Drabinsky M, Braunwald E. Defective cardiac parasympathetic control in patients with heart disease. N Engl J Med, 1971,285(16):877–883
Pessoa L, McMenamin B. Dynamic Networks in the Emotional Brain. Neuroscientist, 2017,23(4):383–396
Naliboff BD, Berman S, Chang L, et al. Sex-related differences in IBS patients: central processing of visceral stimuli. Gastroenterology, 2003,124(7):1738–1747
Wilder-Smith CH, Schindler D, Lovblad K, et al. Brain functional magnetic resonance imaging of rectal pain and activation of endogenous inhibitory mechanisms in irritable bowel syndrome patient subgroups and healthy controls. Gut, 2004,53(11):1595–1601
Naliboff BD, Derbyshire SW, Munakata J, et al. Cerebral activation in patients with irritable bowel syndrome and control subjects during rectosigmoid stimulation. Psychosom Med, 2001,63(3):365–375
Agostini A, Filippini N, Benuzzi F, et al. Functional magnetic resonance imaging study reveals differences in the habituation to psychological stress in patients with Crohn’s disease versus healthy controls. J Behav Med, 2013,36(5):477–487
Jiang F, Yu C, Zuo MJ, et al. Frequency-dependent neural activity in primary angle-closure glaucoma. Neuropsychiatr Dis Treat, 2019,15:271–282
Chen W, Zhang L, Xu Y G, et al. Primary angle-closure glaucomas disturb regional spontaneous brain activity in the visual pathway: an fMRI study. Neuropsychiatr Dis Treat, 2017,13:1409–1417
Di Ciò F, Garaci F, Minosse S, et al. Reorganization of the structural connectome in primary open angle Glaucoma. Neuroimage Clin, 2020,28:102419
Li T, Liu Z, Li J, et al. Altered amplitude of low-frequency fluctuation in primary open-angle glaucoma: a resting-state FMRI study. Invest Ophthalmol Vis Sci, 2014,56(1):322–329
Goel M, Picciani RG, Lee RK, et al. Aqueous humor dynamics: a review. Open Ophthalmol J, 2010,4:52–59
McDougal DH, Gamlin PD. Autonomic control of the eye. Compr Physiol, 2015,5(1):439–473
Kozicz T, Bittencourt JC, May PJ, et al. The Edinger-Westphal nucleus: a historical, structural, and functional perspective on a dichotomous terminology. J Comp Neurol, 2011,519(8):1413–1434
Ruskell GL. Sympathetic innervation of the ciliary muscle in monkeys. Exp Eye Res, 1973,16(3):183–190
Selbach JM, Gottanka J, Wittmann M, et al. Efferent and afferent innervation of primate trabecular meshwork and scleral spur. Invest Ophthalmol Vis Sci, 2000,41(8):2184–2191
Yang F, Zhu X, Liu X, et al. Anatomical evidence for the efferent pathway from the hypothalamus to autonomic innervation in the anterior chamber structures of eyes. Exp Eye Res, 2021,202:108367
LeDoux JE. Emotion circuits in the brain. Annu Rev Neurosci, 2000,23:155–184
Jia F, Lv P, Miao H, et al. Optimization of the Fluorescent Protein Expression Level Based on Pseudorabies Virus Bartha Strain for Neural Circuit Tracing. Front Neuroanat, 2019,13:63
Liu H, Zhu X, Ling Y, et al. Anatomic Evidence for Information Exchange between Primary Afferent Sensory Neurons Innervating the Anterior Eye Chamber and the Dura Mater in Rat. Invest Ophthalmol Vis Sci, 2018,59(8):3424–3430
Isakova LS, Danilov GE, Egorkina SB, et al. Hormonal homeostasis and intraocular pressure in chronic emotional stress caused by influences acting on the amygdala. Fiziol Zh SSSR Im I M Sechenova (Russian), 1989,75(1):124–130
Egorkina SB, Danilov GE. Changes in intraocular pressure in response to experimental testing of the amygdaloid complex. Fiziol Zh SSSR Im I M Sechenova (Russian), 1985,71(6):714–718
Samuels BC, Hammes NM, Johnson PL, et al. Dorsomedial/Perifornical hypothalamic stimulation increases intraocular pressure, intracranial pressure, and the translaminar pressure gradient. Invest Ophthalmol Vis Sci, 2012,53(11):7328–7335
Myagkov AV, Danilov GE, Fatykhov IR. The correcting influence of the locus ceruleus on ophthalmic hypertension of hypothalamic origin. Neurosci Behav Physiol, 2004,34(1):97–100
Martin EI, Ressler KJ, Binder E, et al. The neurobiology of anxiety disorders: brain imaging, genetics, and psychoneuroendocrinology. Clin Lab Med, 2010,30(4):865–891
Hoehn-Saric R, McLeod DR, Funderburk F, et al. Somatic symptoms and physiologic responses in generalized anxiety disorder and panic disorder: an ambulatory monitor study. Arch Gen Psychiatry, 2004, 61(9):913–921
Wang J, Li T, Sabel BA, et al. Structural brain alterations in primary open angle glaucoma: a 3T MRI study. Sci Rep, 2016,6:18969
Burbridge S, Stewart I, Placzek M, Development of the Neuroendocrine Hypothalamus. Compr Physiol, 2016,6(2):623–643
Benarroch EE, The central autonomic network: functional organization, dysfunction, and perspective. Mayo Clin Proc, 1993,68(10):988–1001
Bruinstroop E, Fliers E, Kalsbeek A. Hypothalamic control of hepatic lipid metabolism via the autonomic nervous system. Best Pract Res Clin Endocrinol Metab, 2014,28(5):673–684
Coote J H. A role for the paraventricular nucleus of the hypothalamus in the autonomic control of heart and kidney. Exp Physiol, 2005,90(2):169–173
Guyenet PG. The sympathetic control of blood pressure. Nat Rev Neurosci, 2006,7(5):335–346
Gloster J, Greaves DP. Effect of diencephalic stimulation upon intra-ocular pressure. Br J Ophthalmol, 1957,41(9): 513–532
Von Sallmann L, Lowenstein O. Responses of intraocular pressure, blood pressure, and cutaneous vessels to electric stimulation in the diencephalon: the ninth Francis I. Proctor Lecture. Am J Ophthalmol, 1955,39(4 Pt 2):11–29
Schmerl E, Steinberg B, The role of the diencephalon in regulating ocular tension. Am J Ophthalmol, 1948,31(2):155–158
Gloster J, Greaves DP. Some ocular effects of diencephalic stimulation in the experimental animal. Proc R Soc Med, 1956,49(9):675–680
Schemerl E, Steinberg B. Separation of diencephalic centers concerned with pupillary motility and ocular tension. Am J Ophthalmol, 1950,33(9):1379–1381
Meijer JH, Rietveld WJ, Neurophysiology of the suprachiasmatic circadian pacemaker in rodents. Physiol Rev, 1989,69(3):671–707
Cox CE, Fitzgerald CR, King RL. A preliminary report on the supraoptic nucleus and control of intraocular pressure. Invest Ophthalmol, 1975, 4(1):26–28
Yoshizawa T. New experimental model system to study central regulation of intraocular pressure. Jpn J Ophthalmol, 1993,37(1):9–15
Gong JL, Lou XT, Yuan YX, et al. The increased expression of GABA receptors within the arcuate nucleus is associated with high intraocular pressure. Mol Vis, 2018,24:574–586
Jin J, Xu GX, Yuan ZL. Influence of the hypothalamic arcuate nucleus on intraocular pressure and the role of opioid peptides. PLoS One, 2014,9(4):e82315
Allen GV, Cechetto DF. Functional and anatomical organization of cardiovascular pressor and depressor sites in the lateral hypothalamic area. II. Ascending projections. J Comp Neurol, 1993,330(3):421–438
Renaud LP, Hopkins DA. Amygdala afferents from the mediobasal hypothalamus: an electrophysiological and neuroanatomical study in the rat. Brain Res, 1977, 121(2):201–213
Leonard CM, Scott JW. Origin and distribution of the amygdalofugal pathways in the rat: an experimental neuroanatomical study. J Comp Neurol, 1971,141(3):313–329
Myers B, Mark Dolgas C, Kasckow J, et al. Central stress-integrative circuits: forebrain glutamatergic and GABAergic projections to the dorsomedial hypothalamus, medial preoptic area, and bed nucleus of the stria terminalis. Brain Struct Funct, 2014,219(4):1287–1303
Ulrich-Lai YM, Jones KR, Ziegler DR, et al. Forebrain origins of glutamatergic innervation to the rat paraventricular nucleus of the hypothalamus: differential inputs to the anterior versus posterior subregions. J Comp Neurol, 2011,519(7):1301–1319
Takeuchi Y, Fukui Y, Ichiyama M, et al. Direct amygdaloid projections to the superior salivatory nucleus: a light and electron microscopic study in the cat. Brain Res Bull, 1991,27(1):85–92
Dos Santos Júnior ED, Da Silva AV, Da Silva KR, et al. The centrally projecting Edinger-Westphal nucleus—I: Efferents in the rat brain. J Chem Neuroanat, 2015,68: 22–38
Méndez-Ruette M, Linsambarth S, Moraga-Amaro R, et al. The Role of the Rodent Insula in Anxiety. Front Physiol, 2019,10:330
Kim SY, Adhikari A, Lee SY, et al. Diverging neural pathways assemble a behavioural state from separable features in anxiety. Nature, 2013,496(7444):219–223
Degroot A, Kashluba S, Treit D. Septal GABAergic and hippocampal cholinergic systems modulate anxiety in the plus-maze and shock-probe tests. Pharmacol Biochem Behav, 2001,69(3–4):391–399
Mitra A, Lenglos C, Timofeeva E. Inhibition in the lateral septum increases sucrose intake and decreases anorectic effects of stress. Eur J Neurosci, 2015,41(4):420–433
Buhle JT, Kober H, Ochsner KN, et al. Common representation of pain and negative emotion in the midbrain periaqueductal gray. Soc Cogn Affect Neurosci, 2013,8(6):609–616
Allen GV, Saper CB, Hurley KM, et al. Organization of visceral and limbic connections in the insular cortex of the rat. J Comp Neurol, 1991,311(1):1–16
Sripanidkulchai K, Sripanidkulchai B, Wyss JM. The cortical projection of the basolateral amygdaloid nucleus in the rat: a retrograde fluorescent dye study. J Comp Neurol, 1984,229(3):419–431
Yasui Y, Breder CD, Saper CB, et al. Autonomic responses and efferent pathways from the insular cortex in the rat. J Comp Neurol, 1991,303(3):355–374
Benarroch EE. Periaqueductal gray: an interface for behavioral control. Neurology, 2012,78(3):210–217
Kubo T, Kanaya T, Numakura H, et al. The lateral septal area is involved in mediation of immobilization stress-induced blood pressure increase in rats. Neurosci Lett, 2002,318(1):25–28
Author information
Authors and Affiliations
Corresponding authors
Ethics declarations
On behalf of all authors, the corresponding author states that there is no conflict of interest regarding the publication of this article.
Rights and permissions
About this article
Cite this article
Ma, L., Yang, F., Liu, Q. et al. Anatomical Evidence for the Neural Connection from the Emotional Brain to Autonomic Innervation in the Anterior Chamber Structures of the Eye. CURR MED SCI 42, 417–425 (2022). https://doi.org/10.1007/s11596-022-2571-y
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11596-022-2571-y