Summary
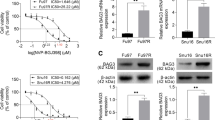
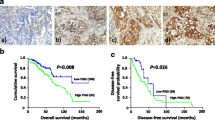
Block of proliferation 1 (BOP1) is a key protein involved in ribosome maturation and affects cancer progression. However, its role in gastric cancer (GC) remains unknown. This study aimed to explore the expression of BOP1 in GC and its potential mechanisms in regulating GC growth, and the relationship between BOP1 level in cancer tissues and survival was also analyzed. The expression of BOP1 was examined by immunohistochemistry (IHC) in a cohort containing 387 patients with primary GC. Cultured GC cells were treated by siRNA to knock down the BOP1 expression, and examined by CCK-8 assay and plate clone formation to assess cell proliferation in vitro. Apoptotic rate of cultured GC cells was detected by flow cytometry with double staining of AnnxinV/PI. The xenografted mouse model was used to assess GC cell proliferation in vivo. Western blot and IHC were also performed to detect the expression levels of BOP1, p53 and p21. Patients with higher level of BOP1 in cancer tissues had significantly poorer survival. BOP1 silencing significantly suppressed GC cell proliferation both in vitro and in vivo. It blocked cell cycle at G0/G1 phase and led to apoptosis of GC cells via upregulating p53 and p21. BOP1 silencing-induced suppression of cell proliferation was partly reversed by pifithrin-α (a p53 inhibitor). Our study demonstrated that BOP1 up-regulation may be a hallmark of GC and it may regulate proliferation of GC cells by activating p53. BOP1 might be considered a novel biomarker of GC proliferation, and could be a potential indicator of prognosis of GC patients. BOP1 might also be a potential target for the treatment of GC patients if further researched.
Similar content being viewed by others
References
Shen L, Shan YS, Hu HM, et al. Management of gastric cancer in Asia: resource-stratified guidelines. Lancet Oncol, 2013,14(12):e535–547
Li P, Huang CM, Zheng CH, et al. Comparison of gastric cancer survival after R0 resection in the US and China. J Surg Oncol, 2018,118(6):975–982
Barry KH, Moore LE, Sampson JN, et al. Prospective study of DNA methylation at chromosome 8q24 in peripheral blood and prostate cancer risk. Br J Cancer, 2017,116(11):1470–1479
Li L, Lv L, Liang Y, et al. Association of 8q23–24 region (8q23.3 loci and 8q24.21 loci) with susceptibility to colorectal cancer: a systematic and updated meta-analysis. Int J Clin Exp Med, 2015,8(11):21001–21013
Shi J, Zhang Y, Zheng W, et al. Fine-scale mapping of 8q24 locus identifies multiple independent risk variants for breast cancer. Int J Cancer, 2016,139(6):1303–1317
Zhi P, Shi J, Liu F. Genetic variations at 8q24 and gastric cancer susceptibility: A meta-analysis study. PLoS One, 2017,12(12):e0188774
Wang X, Liu Y, Shao D, et al. Recurrent amplification of MYC and TNFRSF11B in 8q24 is associated with poor survival in patients with gastric cancer. Gastric Cancer, 2016,19(1):116–127
Strezoska Z, Pestov DG, Lau LF. Bop1 is a mouse WD40 repeat nucleolar protein involved in 28S and 5. 8S RRNA processing and 60S ribosome biogenesis. Mol Cell Biol, 2000,20(15):5516–5528
Strezoska Z, Pestov DG, Lau LF. Functional inactivation of the mouse nucleolar protein Bop1 inhibits multiple steps in pre-rRNA processing and blocks cell cycle progression. J Biol Chem, 2002,277(33):29617–29625
Chung KY, Cheng IK, Ching AK, et al. Block of proliferation 1 (BOP1) plays an oncogenic role in hepatocellular carcinoma by promoting epithelial-to-mesenchymal transition. Hepatology, 2011,54(1):307–318
Qi J, Yu Y, Akilli Ozturk O, et al. New Wnt/beta-catenin target genes promote experimental metastasis and migration of colorectal cancer cells through different signals. Gut, 2016,65(10):1690–1701
Pestov DG, Strezoska Z, Lau LF. Evidence of p53-dependent cross-talk between ribosome biogenesis and the cell cycle: effects of nucleolar protein Bop1 on G(1)/S transition. Mol Cell Biol, 2001,21(13):4246–4255
Liu R, Iadevaia V, Averous J, et al. Impairing the production of ribosomal RNA activates mammalian target of rapamycin complex 1 signalling and downstream translation factors. Nucleic Acids Res, 2014,42(8):5083–5096
Evans DS, Kapahi P, Hsueh WC, et al. TOR signaling never gets old: aging, longevity and TORC1 activity. Ageing Res Rev, 2011,10(2):225–237
Takada H, Kurisaki A. Emerging roles of nucleolar and ribosomal proteins in cancer, development, and aging. Cell Mol Life Sci, 2015,72(21):4015–4025
Rubbi CP, Milner J. Disruption of the nucleolus mediates stabilization of p53 in response to DNA damage and other stresses. EMBO J, 2003,22(22):6068–6077
Rohrmoser M, Holzel M, Grimm T, et al. Interdependence of Pes1, Bop1, and WDR12 controls nucleolar localization and assembly of the PeBoW complex required for maturation of the 60S ribosomal subunit. Mol Cell Biol, 2007,27(10):3682–3694.
James A, Wang Y, Raje H, et al. Nucleolar stress with and without p53. Nucleus, 2014,5(5):402–426
Scala F, Brighenti E, Govoni M, et al. Direct relationship between the level of p53 stabilization induced by rRNA synthesis-inhibiting drugs and the cell ribosome biogenesis rate. Oncogene, 2016,35(8):977–989
Vikhanskaya F, Siddique MM, Kei Lee M, et al. Evaluation of the combined effect of p53 codon 72 polymorphism and hotspot mutations in response to anticancer drugs. Clin Cancer Res, 2005,11(12):4348–4356
Tran TQ, Lowman XH, Reid MA, et al. Tumor-associated mutant p53 promotes cancer cell survival upon glutamine deprivation through p21 induction. Oncogene, 2017,36(14):1991–2001
Rasul A, Ding C, Li X, et al. Dracorhodin perchlorate inhibits PI3K/Akt and NF-kappaB activation, up-regulates the expression of p53, and enhances apoptosis. Apoptosis, 2012,17(10):1104–1119
Bursac S, Brdovcak MC, Pfannkuchen M, et al. Mutual protection of ribosomal proteins L5 and L11 from degradation is essential for p53 activation upon ribosomal biogenesis stress. Proc Natl Acad Sci USA, 2012,109(50):20467–20472
Jin A, Itahana K, O’Keefe K, et al. Inhibition of HDM2 and activation of p53 by ribosomal protein L23. Mol Cell Biol, 2004,24(17):7669–7680
Bornkamm GW, Berens C, Kuklik-Roos C, et al. Stringent doxycycline-dependent control of gene activities using an episomal one-vector system. Nucleic Acids Res, 2005,33(16):e137
Acknowledgments
We extend our sincere gratitude to all staff of NHC Key Laboratory of Glycoconjugates Research of Fudan University.
Author information
Authors and Affiliations
Corresponding author
Additional information
Conflict of Interest Statement
The authors declare that they have no conflicts of interest.
This study was supported by National Natural Science Foundation of China (No. 31670806).
Electronic supplementary material
Rights and permissions
About this article
Cite this article
Yang, Yp., Qin, Rh., Zhao, Jj. et al. BOP1 Silencing Suppresses Gastric Cancer Proliferation through p53 Modulation. CURR MED SCI 41, 287–296 (2021). https://doi.org/10.1007/s11596-021-2345-y
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11596-021-2345-y