Abstract
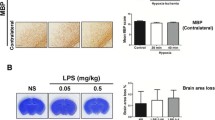
Activated protein C (APC), a natural anticoagulant, has been reported to exert direct vasculoprotective, neural protective, anti-inflammatory, and proneurogenic activities in the central nervous system. This study was aimed to explore the neuroprotective effects and potential mechanisms of APC on the neurovascular unit of neonatal rats with intrauterine infection-induced white matter injury. Intraperitoneal injection of 300 μg/kg lipopolysaccharide (LPS) was administered consecutively to pregnant Sprague-Dawley rats at embryonic days 19 and 20 to establish the rat model of intrauterine infection- induced white matter injury. Control rats were injected with an equivalent amount of sterile saline on the same time. APC at the dosage of 0.2 mg/kg was intraperitoneally injected to neonatal rats immediately after birth. Brain tissues were collected at postnatal day 7 and stained with hematoxylin and eosin (H&E). Immunohistochemistry was used to evaluate myelin basic protein (MBP) expression in the periventricular white matter region. Blood-brain barrier (BBB) permeability and brain water content were measured using Evens Blue dye and wet/dry weight method. Double immunofluorescence staining and real-time quantitative PCR were performed to detect microglial activation and the expression of protease activated receptor 1 (PAR1). Typical pathological changes of white matter injury were observed in rat brains exposed to LPS, and MBP expression in the periventricular region was significantly decreased. BBB was disrupted and the brain water content was increased. Microglia were largely activated and the mRNA and protein levels of PAR1 were elevated. APC administration ameliorated the pathological lesions of the white matter and increased MBP expression. BBB permeability and brain water content were reduced. Microglia activation was inhibited and the PAR1 mRNA and protein expression levels were both down-regulated. Our results suggested that APC exerted neuroprotective effects on multiple components of the neurovascular unit in neonatal rats with intrauterine infection- induced white matter injury, and the underlying mechanisms might involve decreased expression of PAR1.
Similar content being viewed by others
References
Back SA. Perinatal white matter injury: the changing spectrum of pathology and emerging insights into pathogenetic mechanisms. Ment Retard Dev Disabil Res Rev, 2006,12(2):129–140
Haynes RL, Sleeper LA, Volpe JJ, et al. Neuropathologic studies of the encephalopathy of prematurity in the late preterm infant. Clin Perinatol, 2013,40(4):707–722
Volpe JJ. Systemic inflammation, oligodendroglial maturation, and the encephalopathy of prematurity. Ann Neurol, 2011,70(4):525–529
Vangilder RL, Rosen CL, Barr TL, et al. Targeting the neurovascular unit for treatment of neurological disorders. Pharmacol Ther, 2011,130(3):239–247
Rezaie AR. Regulation of the protein C anticoagulant and antiinflammatory pathways. Curr Med Chem, 2010,17(19): 2059–2069
Zlokovic BV, Griffin JH. Cytoprotective protein C pathways and implications for stroke and neurological disorders. Trends Neurosci, 2011,34(4):198–209
Mosnier LO, Zlokovic BV, Griffin JH. Cytoprotective-selective activated protein C therapy for ischaemic stroke. Thromb Haemost, 2014,112(5):883–892
Maggio N, Itsekson Z, Ikenberg B, et al. The anticoagulant activated protein C (aPC) promotes metaplasticity in the hippocampus through an EPCR-PAR1-S1P1 receptors dependent mechanism. Hippocampus, 2014,24(8):1030–1038
Gorbacheva L, Strukova S, Pinelis V, et al. NF-?B-dependent and -independent pathways in the protective effects of activated protein C in hippocampal and cortical neurons at excitotoxicity. Neurochem Int, 2013,63(2):101–111
Guo H, Zhao Z, Yang Q, et al. An activated protein C analog stimulates neuronal production by human neural progenitor cells via a PAR1-PAR3-S1PR1-Akt pathway. J Neurosci, 2013,33(14):6181–6190
Rousset CI, Chalon S, Cantagrel S, et al. Maternal exposure to LPS induces hypomyelination in the internal capsule and programmed cell death in the deep gray matter in newborn rats. Pediatr Res, 2006,59(3):428–433
Yesilirmak DC, Kumral A, Baskin H, et al. Activated protein C reduces endotoxin-induced white matter injury in the developing rat brain. Brain Res, 2007,1164:14–23
Kuypers E, Ophelders D, Jellema RK, et al. White matter injury following fetal inflammatory response syndrome induced by chorioamnionitis and fetal sepsis: lessons from experimental ovine models. Early Hum Dev, 2012,88(12): 931–936
Malaeb S, Dammann O. Fetal inflammatory response and brain injury in the preterm newborn. J Child Neurol, 2009,24(9):1119–1126
Dammann O, Leviton A. Intrauterine infection, cytokines, and brain damage in the preterm newborn. Pediatr Res, 1997,42 (1):1–8
Hagberg H, Mallard C, Jacobsson B. Role of cytokines in preterm labour and brain injury. BJOG, 2005,112(Suppl): 16–18
Li J, Baud O, Vartanian T, et al. Peroxynitrite generated by inducible nitric oxide synthase and NADPH oxidase mediates microglial toxicity to oligodendrocytes. Proc Natl Acad Sci USA, 2005,102 (28):9936–9941
Deane R, LaRue B, Sagare AP, et al. Endothelial protein C receptor-assisted transport of activated protein C across the mouse blood–brain barrier. J Cereb Blood Flow Metab, 2009,29(1):25–33
Coughlin SR. Thrombin signaling and protease-activated receptors. Nature, 2000,407(6801):258–264
Feistritzer C, Riewald M. Endothelial barrier protection by activated protein C through PAR1-dependent sphingosine-1-phosphate receptor-1 crossactivation. Blood, 2005, 105(8):3178–3184
Yang L, Bae JS, Manithody C, et al. Identification of a specific exosite on activated protein C for interaction with protease-activated receptor 1. J Biol Chem, 2007,282(35): 25 493–25 500
Harry GJ, Kraft AD. Microglia in the developing brain: a potential target with lifetime effects. Neurotoxicology, 2012,33(2):191–206
Vangilder RL, Rosen CL, Barr TL, et al. Targeting the neurovascular unit for treatment of neurological disorders. Pharmacol Ther, 2011,130(3):239–247
Deng W, Pleasure J, Pleasure D. Progress in periventricular leukomalacia. Arch Neurol, 2008,65(10):1291–1295
Lee CY, Landreth GE. The role of microglia in amyloid clearance from the AD brain. J Neural Transm, 2010,117(8):949–960
Joyce DE, Grinnell BW. Recombinant human activated protein C attenuates the inflammatory response in endothelium and monocytes by modulating nuclear factor-kappaB. Crit Care Med, 2002,30 (5 Suppl): S288–S293
Riewald M, Petrovan RJ, Donner A, et al. Activation of endothelial cell protease activated receptor 1 by the protein C pathway. Science, 2002,296(5574):1880–1882
Author information
Authors and Affiliations
Corresponding author
Additional information
This project was supported by grants from National Natural Science Foundation of China (No. 81471519 and No. 81401277) and the Program for Changjiang Scholars and Innovative Research Team in University of China (No. IRT_14R20)
Rights and permissions
About this article
Cite this article
Jin, Sj., Liu, Y., Deng, Sh. et al. Protective effects of activated protein C on neurovascular unit in a rat model of intrauterine infection-induced neonatal white matter injury. J. Huazhong Univ. Sci. Technol. [Med. Sci.] 35, 904–909 (2015). https://doi.org/10.1007/s11596-015-1526-y
Received:
Revised:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11596-015-1526-y