Summary
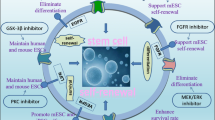
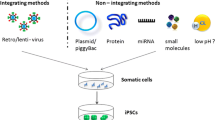
Induced pluripotent stem cells (iPSCs) can be propagated indefinitely, while maintaining the capacity to differentiate into all cell types in the body except for the extra-embryonic tissues. This iPSC technology not only represents a new way to use individual-specific stem cells for regenerative medicine but also constitutes a novel method to obtain large numbers of disease-specific cells for biomedical research. However, the low efficiency of reprogramming and genomic integration of oncogenes and viral vectors limit the potential application of iPSCs. Chemical-induced reprogramming offers a novel approach to generating iPSCs. In this study, a new combination of small-molecule compounds (SMs) (sodium butyrate, A-83-01, CHIR99021, Y-27632) under conditions of transient folate deprivation was used to generate iPSC. It was found that transient folate deprivation combined with SMs was sufficient to permit reprogramming from mouse embryonic fibroblasts (MEFs) in the presence of transcription factors, Oct4 and Klf4, within 25 days, replacing Sox2 and c-Myc, and accelerated the generation of mouse iPSCs. The resulting cell lines resembled mouse embryonic stem (ES) cells with respect to proliferation rate, morphology, pluripotency-associated markers and gene expressions. Deprivation of folic acid, combined with treating MEFs with SMs, can improve the inducing efficiency of iPSCs and reduce their carcinogenicity and the use of exogenous reprogramming factors.
Similar content being viewed by others
References
Takahashi K, Yamanaka S. Induction of pluripotent stem cells from mouse embryonic and adult fibroblast cultures by defined factors. Cell, 2006,126(4):663–676
Majad K, Karthikeyan N, Hongfang L, et al. Delivery of reprogramming factors into fibroblasts for generation of non-genetic induced pluripotent stem cells using a cationic bolaamphiphile as a non-viral vector. Biomaterials, 2013,34(21):5336–5343
Park IH, Arora N, Huo H, et al. Disease-specific induced pluripotent stem cells. Cell, 2008,134(5):877–886
Lin SY, Lee WR, Su YF, et al. Folic acid inhibits endothelial cell proliferation through activating the cSrc/ERK 2/NF-κB/p53 pathway mediated by folic acid receptor. Angiogenesis, 2012,15(4):671–683
Hsu SP, Lee WS. Progesterone receptor activation of extranuclear signaling pathways in regulating p53 expression in vascular endothelial cells. Mol Endocrinol, 2011,25(3):421–432
Hong H, Takahashi K, Ichisaka T, et al. Suppression of induced pluripotent stem cell generation by the p53-p21 pathway. Nature, 2009,460(7259):1132–1135
Kawamura T, Suzuki J, Wang YV, et al. Linking the p53 tumour suppressor pathway to somatic cell reprogramming. Nature, 2009,460(7259):1140–1144
Li H, Collado M, Villasante A, et al. The Ink/Arf locus is a barrier for iPS cell reprogramming. Nature, 2009, 460(7259):1136–1139
Marion RM, Strati K, Li H, et al. A p53-mediated DNA damage response limits reprogramming to ensure iPS cell genomic integrity. Nature, 2009,460(7259):1149–1153
Utikal J, Polo JM, Stadtfeld M, et al. Immortalization eliminates a roadblock during celluar reprogramming into iPS cells. Nature, 2009,460(7259):1145–1148
Yan QY, Qiu ZD, Hu WT, et al. An inducing system for differentiation of folate-deficient mouse embryo fibroblasts to pluripotent stem cells. Acta Med Univ Sci Technol Huazhong (Chinese), 2013,42(2):192–194
Xu Y, Wei X, Wang M, et al. Proliferation rate of somatic cells affects reprogramming efficiency. J Biol Chem, 2013,288(14):9767–9778
Martinez-Fernandez A, Nelson TJ, A Terzic A, et al. Nuclear reprogramming strategy modulates differentiation potential of induced pluripotent stem cells. J Cardiovasc TransI Res, 2011,4(2):131–137
Zhang Y, Li WL, Laurent T, et al. Small molecules, big roles—the chemical manipulation of stem cell fate and somatic cell reprogramming. J Cell Sci, 2012,125(Pt23):5609–5620
Zhang R, Zhang LH, Xie X, et al. iPSCs and small molecules: a reciprocal effort towards better approaches for drug discovery. Acta Pharmacologica Sinica, 2013,34(6):765–776
Huangfu D, Maehr R, Guo W, et al. Induction of pluripotent stem cells by defined factors is greatly improved by small-molecule compounds. Nat Biotechnol, 2008,26(7):795–797
Mali P, Chou BK, Yen J, et al. Butyrate greatly enhances derivation of human induced pluripotent stem cells by promoting epigenetic remodeling and the expression of pluripotency-associated genes. Stem Cells, 2010,28(4):713–720
Liang G, Taranova O, Xia K, et al. Butyrate promotes induced pluripotent stem cell generation. J Biol Chem, 2010,285(33):25516–25521
Zhang Z, Gao Y, Gordon A, et al. Efficient generation of fully reprogrammed human iPS cells via polycistronic retroviral vector and a new cocktail of chemical compounds. PLoS One, 2011,6(10):e26592
Li R, Liang J, Ni S, et al. A mesenchymal-to-epithelial transition initiates and is required for the nuclear reprogramming of mouse fibroblasts. Cell Stem Cell, 2010,7(1):51–63
Li W, Wei W, Zhu S, et al. Generation of rat and human induced pluripotent stem cells by combining genetic reprogramming and chemical inhibitors. Cell Stem Cell, 2009,4(1):16–19
Li W, Zhou H, Abujarour R, et al. Generation of human-induced pluripotent stem cells in the absence of exogenous Sox2. Stem Cells, 2009,27(12):2992–3000
Choi YJ, Lin CP, Ho JJ, et al. miR-34 miRNAs provide a barrier for somatic cell reprogramming. Nat Cell Biol, 2011,13(11):1353–1360
Ye D, Wang G, Liu Y, et al. MiR-138 promotes induced pluripotent stem cell generation through the regulation of the P53 Signaling. Stem Cells, 2012,30(8):1645–1654
Author information
Authors and Affiliations
Corresponding author
Additional information
This project was supported by grants from the National Natural Science Foundation of China (No. 81271407), and the Chinese Postdoctoral Scientific Research Fund (No. 20110490453).
Rights and permissions
About this article
Cite this article
Hu, Wt., Yan, Qy., Fang, Y. et al. Transient folate deprivation in combination with small-molecule compounds facilitates the generation of somatic cell-derived pluripotent stem cells in mice. J. Huazhong Univ. Sci. Technol. [Med. Sci.] 34, 151–156 (2014). https://doi.org/10.1007/s11596-014-1249-5
Received:
Revised:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11596-014-1249-5