Abstract
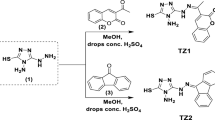
The inhibition capacity of carbon steel (CS) in 0.5M H2SO4 by three 8-hydroxyquinoline grafted triazole derivatives (EHTC, AHTC, and MHTC) have been investigated using electrochemical impedance spectroscopy (EIS), potentiodynamic polarization (PPD), and weight loss measurements (WLM) at 298 K. Generally, the results clearly show that the inhibition performance (η %) increases with an increase in the concentration of EHTC, MHTC, and AHTC, reaching a maximum value of 95.5% (EHTC), 95.1% (MHTC), and 94.1% (AHTC) at the optimal concentration (10−3 M) for PPD technical. The PPD shows that EHTC, AHTC, and MHTC behave as mixed-type inhibitors. In addition, the inhibitor obeys the single layer adsorption isotherm of Langmuir. Scanning electron microscopy (SEM) analysis of the CS has also been investigated and discussed. The theoretical calculations and MD simulations show a better correlation with the experimental results for the studied triazole derivatives.

















Similar content being viewed by others
References
Ji G, Shukla SK, Dwivedi P, Sundaram S, Prakash R (2011) Ind Eng Chem Res 50:11954–11959
Singh AK, Quraishi MA (2010) Effect of Cefazolin on the corrosion of mild steel in HCl solution. Corros Sci 52:152–160
El Azzouzi M, Aouniti A, Tighadouin S, Elmsellem H, Radi S, Hammouti B, El Assyry A, Bentiss F, Zarrouk A (2016) Some hydrazine derivatives as corrosion inhibitors for mild steel in 1.0M HCl: Weight loss, electrochemichal, SEM and theoretical studies. J Mol Liq 221:633–641
Zarrouk A, Zarrok H, Ramli Y, Bouachrine M, Hammouti B, Sahibed-dine A, Bentiss F (2016) Inhibitive properties, adsorption and theoretical study of 3,7-dimethyl-1-(prop-2-yn-1-yl)quinoxalin-2(1H)-one as efficient corrosion inhibitor for carbon steel in hydrochloric acid solution. J Mol Liq 222:239–252
Tayebi H, Bourazmi H, Himmi B, El Assyry A, Ramli Y, Zarrouk A, Geunbour A, Hammouti B, Ebenso EE (2014) Der Pharm Lett 6(6):20–34
El Hezzat M, Assouag M, Zarrok H, Benzekri Z, El Assyry A, Boukhris S, Souizi A, Galai M, Touir R, Ebn Touhami M, Oudda H, Zarrouk A (2015) Der Pharma Chem 7(10):77–88
Fouda AS, Al-Sarawy AA, El-Katori EE (2006) Pyrazolone derivatives as corrosion inhibitors for C-steel in hydrochloric acid solution. Desalination 201:1–13
Oguzie EE, Li Y, Wang FH (2007) Electrochim Acta 53:909–914
Achary G, Sachin HP, Naik YA, Venkatesha TV (2008) Mater Chem Phys 107:44–50
Ashassi-Sorkhabi H, Ghalebsaz-Jeddi N (2005) Inhibition effect of polyethylene glycol on the corrosion of carbon steel in sulphuric acid. Mater Chem Phys 92:480–486
Boumhara K, Tabyaoui M, Jama C, Bentiss F (2015) Artemisia Mesatlantica essential oil as green inhibitor for carbon steel corrosion in 1M HCl solution: Electrochemical and XPS investigations. J Ind Eng Chem 29:146–155
Elkhotfi Y, Forsal I, Rakib EM, Mernari B (2018) Port Electrochim Acta 36:77–87
Verma C, Olasunkanmi LO, Ebenso EE, Quraishi MA (2018) J Mol Liq. 251:100–118
About H, El Faydy M, Benhiba F, Rouifi Z, Boudalia M, Guenbour A, Zarrok H, Lakhrissi B, Oudda H, Warad I, Zarrouk A (2019) J Bio-Tribo-Corrosion 5. https://doi.org/10.1007/s40735-019-0233-9
El Faydy M, Touir R, Ebn Touhami M, Zarrouk A, Jama C, Lakhrissi B, Olasunkanmi LO, Ebenso EE, Bentiss F (2018) Corrosion inhibition performance of newly synthesized 5-alkoxymethyl-8-hydroxyquinoline derivatives for carbon steel in 1 M HCl solution: experimental, DFT and Monte Carlo simulation studies. Phys Chem Chem Phys 20:20167–20187
Faydy ME, Rbaa M, Lakhrissi L, Lakhrissi B, Warad I, Zarrouk A, Obot IB (2019) Corrosion protection of carbon steel by two newly synthesized benzimidazol-2-ones substituted 8-hydroxyquinoline derivatives in 1 M HCl: Experimental and theoretical study. Surf Interfaces 14:222–237
Rouifi Z, Benhiba F, Faydy ME, Laabaissi T, About H, Oudda H, Warad I, Guenbour A, Lakhrissi B, Zarrouk A (2019) Performance and computational studies of new soluble triazole as corrosion inhibitor for carbon steel in HCl. Chem Data Collect 22:100242
Rbaa M, Galai M, Abousalem AS, Lakhrissi B, Touhami ME, Warad I, Zarrouk A (2019) Ionics:1–20
Haque J, Verma C, Srivastava V, Quraishi MA, Ebenso EE (2018) Experimental and quantum chemical studies of functionalized tetrahydropyridines as corrosion inhibitors for mild steel in 1 M hydrochloric acid. Results Phys 9:1481–1493
Olasunkanmi LO, Moloto BP, Obot IB, Ebenso EE (2018) Anticorrosion studies of some hydantoin derivatives for mild steel in 0.5 M HCl solution: Experimental, quantum chemical, Monte Carlo simulations and QSAR studies. J Mol Liq 252:62–74
Verma C, Olasunkanmi LO, Quadri TW, Sherif E-SM, Ebenso EE (2018) Gravimetric, Electrochemical, Surface Morphology, DFT, and Monte Carlo Simulation Studies on Three N-Substituted 2-Aminopyridine Derivatives as Corrosion Inhibitors of Mild Steel in Acidic Medium. J Phys Chem C 122:11870–11882
Dagdag O, Safi Z, Erramli H, Cherkaoui O, Wazzan N, Guo L, Verma C, Ebenso EE, El Harfi A (2019) Adsorption and anticorrosive behavior of aromatic epoxy monomers on carbon steel corrosion in acidic solution: computational studies and sustained experimental studies. RSC Adv 9:14782–14796
Zarrouk A, Hammouti B, Dafali A, Bouachrine M, Zarrok H, Boukhris S, Al-Deyab SS (2014) J Saudi Chem Soc. 18:450–455
El-Yaktini A, Lachiri A, El-Faydy M, Benhiba F, Zarrok H, El-Azzouzi M, Zertoubi M, Azzi M, Lakhrissi B, Zarrouk A (2018) Orient J Chem 34:3016–3029
Olasunkanmi LO, Obot IB, Kabanda MM, Ebenso EE (2015) Some Quinoxalin-6-yl Derivatives as Corrosion Inhibitors for Mild Steel in Hydrochloric Acid: Experimental and Theoretical Studies. J Phys Chem C 119:16004–16019
Roque JM, Pandiyan T, Cruz J, García-Ochoa E (2008) Corros Sci 50:614–624
Obot IB, Macdonald DD, Gasem ZM (2015) Corros Sci 99:1–30
Sun H (1998) COMPASS: An ab Initio Force-Field Optimized for Condensed-Phase ApplicationsOverview with Details on Alkane and Benzene Compounds. J Phys Chem B 102:7338–7364
Saha SK, Dutta A, Ghosh P, Sukul D, Banerjee P (2016) Novel Schiff-base molecules as efficient corrosion inhibitors for mild steel surface in 1 M HCl medium: experimental and theoretical approach. Phys Chem Chem Phys 18:17898–17911
Oguzie EE, Oguzie KL, Akalezi CO, Udeze IO, Ogbulie JN, Njoku VO (2013) ACS Sustainable Chem. Eng. 1:214–225
Hegazy MA, El-Tabei AS (2013) Synthesis, Surface Properties, Synergism Parameter and Inhibitive Performance of Novel Cationic Gemini Surfactant on Carbon Steel Corrosion in 1 M HCl Solution. J Surfactants Deterg 16:221–232
Salhi A, Tighadouini S, El-Massaoudi M, Elbelghiti M, Bouyanzer A, Radi S, El Barkany S, Bentiss F, Zarrouk A (2017) Keto-enol heterocycles as new compounds of corrosion inhibitors for carbon steel in 1 M HCl: Weight loss, electrochemical and quantum chemical investigation. J Mol Liq 248:340–349
Ramezanzadeh B, Arman SY, Mehdipour M, Markhali BP (2014) Analysis of electrochemical noise (ECN) data in time and frequency domain for comparison corrosion inhibition of some azole compounds on Cu in 1.0M H2SO4 solution. Appl Surf Sci 289:129–140
Zarrok H, Zarrouk A, Hammouti B, Salghi R, Jama C, Bentiss F (2012) Corrosion control of carbon steel in phosphoric acid by purpald – Weight loss, electrochemical and XPS studies. Corros Sci 64:243–252
Deng Q, Ding N-N, Wei X-L, Cai L, He X-P, Long Y-T, Chen G-R, Chen K (2012) Identification of diverse 1,2,3-triazole-connected benzyl glycoside-serine/threonine conjugates as potent corrosion inhibitors for mild steel in HCl. Corros Sci 64:64–73
Laabaissi T, Lgaz H, Oudda H, Benhiba F, Zarrok H, Zarrouk A, El Midaoui A, Lakhrissi B, Touir R (2017) J Mater Environ Sci 8:1054–1067
Rbaa M, Benhiba F, Obot IB, Oudda H, Warad I, Lakhrissi B, Zarrouk A (2019) Two new 8-hydroxyquinoline derivatives as an efficient corrosion inhibitors for mild steel in hydrochloric acid: Synthesis, electrochemical, surface morphological, UV–visible and theoretical studies. J Mol Liq 276:120–133
Bentiss F, Lebrini M, Lagrenée M, Traisnel M, Elfarouk A, Vezin H (2007) Electrochim Acta 52:6865–6872
Tebbji K, Oudda H, Hammouti B, Benkaddour M, Al-Deyab SS, Aouniti A, Radi S, Ramdani A (2011) The effect of 1′,3,5,5′-tetramethyl-1′H-1,3′-bipyrazole on the corrosion of steel in 1.0 M hydrochloric acid. Res Chem Intermed 37:985–1007
Hsissou R, Benhiba F, Khudhair M, Berradi M, Mahsoune A, Oudda H, El Harfi A, Obot IB, Zarrouk A (2020) J King Saud Univ Sci. 32:667–676
Emregül KC, Hayvalí M (2006) Studies on the effect of a newly synthesized Schiff base compound from phenazone and vanillin on the corrosion of steel in 2M HCl. Corros Sci 48:797–812
Benabdellah M, Touzani R, Dafali A, Hammouti B, El Kadiri S (2007) Ruthenium–ligand complex, an efficient inhibitor of steel corrosion in H3PO4 media. Mater Lett 61:1197–1204
Torres VV, Amado RS, de Sá CF, Fernandez TL, da CA SR, Torres AG, D’ Elia E (2011) Corros Sci 53:2385–2392
Zarrok H, Al-Deyab SS, Zarrouk A, Salghi R, Hammouti B, Oudda H, Bouachrine M, Bentiss F (2012) Int J Electrochem Sci 7:4047–4063
Rbaa M, Lgaz H, El Kacimi Y, Lakhrissi B, Bentiss F, Zarrouk A (2018) Synthesis, characterization and corrosion inhibition studies of novel 8-hydroxyquinoline derivatives on the acidic corrosion of mild steel: Experimental and computational studies. Mater Discover 12:43–54
El Faydy M, Galai M, El Assyry A, Tazouti A, Touir R, Lakhrissi B, Ebn Touhami M, Zarrouk A (2016) Experimental investigation on the corrosion inhibition of carbon steel by 5-(chloromethyl)-8-quinolinol hydrochloride in hydrochloric acid solution. J Mol Liq 219:396–404
Zarrouk A, Hammouti B, Dafali A, Bentiss F (2013) Inhibitive Properties and Adsorption of Purpald as a Corrosion Inhibitor for Copper in Nitric Acid Medium. Ind Eng Chem Res 52:2560–2568
Ouakki M, Rbaa M, Galai M, Lakhrissi B, Rifi EH, Cherkaoui M (2018) Experimental and Quantum Chemical Investigation of Imidazole Derivatives as Corrosion Inhibitors on Mild Steel in 1.0 M Hydrochloric Acid. J Bio-Tribo-Corrosion 4:4. https://doi.org/10.1007/s40735-018-0151-2
Hsissou R, Dagdag O, Abbout S, Benhiba F, Berradi M, El Bouchti M, Berisha A, Hajjaji N, Elharfi A (2019) Novel derivative epoxy resin TGETET as a corrosion inhibition of E24 carbon steel in 1.0 M HCl solution. Experimental and computational (DFT and MD simulations) methods. J Mol Liq 284:182–192
Soro D, Ekou L, Koné MG-R, Ekou T, Ziao N (2019) DFT Study of Molecular Stability and Reactivity on Some Hydroxamic Acids: An Approach by Hirshfeld Population Analysis. Eur J Eng Res Sci 4:45–49
Guo L, Safi ZS, Kaya S, Shi W, Tüzün B, Altunay N, Kaya C (2018) Front Chem 6(155):1–12
Khattabi M, Benhiba F, Tabti S, Djedouani A, El Assyry A, Touzani R, Warad I, Oudda H, Zarrouk A (2019) Performance and computational studies of two soluble pyran derivatives as corrosion inhibitors for mild steel in HCl. J Mol Struct 1196:231–244
Guo A, Duan G, He K, Sun B, Fan C, Hu S (2013) Synergistic effect between 2-oleyl-1-oleylamidoethyl imidazoline ammonium methylsulfate and halide ion by molecular dynamics simulation. Comput Theor Chem 1015:21–26
Xie S-W, Liu Z, Han G-C, Li W, Liu J, Chen Z (2015) Molecular dynamics simulation of inhibition mechanism of 3,5-dibromo salicylaldehyde Schiff’s base. Comput Theor Chem 1063:50–62
Author information
Authors and Affiliations
Corresponding author
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
ESM 1
(DOC 813 kb)
Rights and permissions
About this article
Cite this article
Rouifi, Z., Benhiba, F., El Faydy, M. et al. 8-hydroxyquinoline grafted triazole derivatives as corrosion inhibitors for carbon steel in H2SO4 solution: Electrochemical and theoretical studies. Ionics 27, 2267–2288 (2021). https://doi.org/10.1007/s11581-021-03974-6
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11581-021-03974-6