Abstract
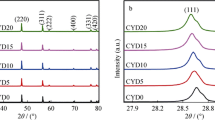
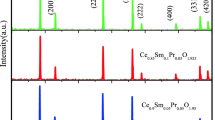
Nanocrystalline co-doped ceria Ce0.8Sm0.2−xYxO2−δ solid electrolytes for intermediate-temperature solid oxide fuel cells (IT-SOFCs) were synthesized through sol–gel auto-combustion method. The prepared samples were sintered via microwave sintering at 1200 °C for 1 h. The X-ray diffraction analysis of co-doped ceria system reveals formation of the samples with a single-phase cubic fluorite structure. The lattice parameter values were calculated from X-ray diffraction patterns. The calculated crystallite sizes of all the samples were found to be in the range of 17 and 28 nm. Surface morphologies and elemental analysis of all the samples were carried out by using SEM and EDS analysis. The existence of chemical bonding in the samples was studied by FTIR spectroscopy. The presence of oxygen vacancies and evaluation of their concentration in the material was carried out using Raman spectroscopy analysis. Electrical properties of all the samples were analyzed by impedance spectroscopy. It was found that microwave sintered co-doped ceria sample Ce0.8Sm0.1Y0.1O2−δ exhibits the highest total ionic conductivity with minimum activation energy among all the compositions and conventional sintered sample. Therefore, it can be concluded that the microwave sintered Ce0.8Sm0.1Y0.1O2−δ sample may be useful as a promising electrolyte material for the IT-SOFCs.









Similar content being viewed by others
References
Inaba H, Tagawa H (1996) Ceria-based solid electrolytes. Solid State Ionics 83:1–16. https://doi.org/10.1016/0167-2738(95)00229-4
Minh NQ (1993) Ceramic fuel cells. J Am Ceramic Society 76:563–588. https://doi.org/10.1111/j.1151-2916.1993.tb03645.x
Choy K, Bai W, Charojrochkul JS, Steele BCH (1998) The development of intermediate-temperature solid oxide fuel cells for the next millennium. J Power Sources 71:361–369. https://doi.org/10.1016/S0378-7753(97)02728-6
Ramesh S, James Raju KC (2012) Preparation and characterization of Ce1–x(Gd0.5Pr0.5)xO2 electrolyte for IT-SOFCs. Int J Hydrog Energy 37:10311–10317. https://doi.org/10.1016/j.ijhydene.2012.04.008
Stojmenovic M, Zunic M, Gulicovski J, B-Bogdanovic D, H-Antunovic I, Dodevskti V, Mentus S (2015) Structural, morphological, and electrical properties of doped ceria as a solid electrolyte for intermediate-temperature solid oxide fuel cells. J Mater Sci 50:3781–3794. https://doi.org/10.1007/s10853-015-8943-y
Khakpour Z, Youzbashi AA, Maghsoudipour A (2014) Influence of Gd3+ and Dy3+ co-doping and sintering regime on enhancement of electrical conductivity of ceria-based solid electrolyte. Ionics 20:1407–1417. https://doi.org/10.1007/s11581-014-1090-7
Fu Y-P, Chen S-H, Huang J-J (2010) Preparation and characterization of Ce0.8M0.2O2–δ (M = Y, Gd, Sm, Nd, La) solid electrolyte materials for solid oxide fuel cells. Int J Hydrog Energy 35:745–752. https://doi.org/10.1016/j.ijhydene.2009.10.093
Pikalova EY, Murashkina AA, Maragou VI, Demin AK, Strekalovsky VN, Tsiakaras PE (2011) CeO2 based materials doped with lanthanides for applications in intermediate temperature electrochemical devices. Int J Hydrog Energy 36:6175–6183. https://doi.org/10.1016/j.ijhydene.2011.01.132
Jaiswal N, Kumar D, Upadhyay S, Parkash O (2015) Preparation and characterization of Ce0.85La0.15−xSrxO{2−(0.075+x/2)} solid electrolytes for intermediate temperature solid oxide fuel cells. Ionics 21:497–505. https://doi.org/10.1007/s11581-014-1190-4
Padmasree KP, M-Lozano RA, Montemayor SM, Fuentes AF (2011) Electrical conduction and dielectric relaxation process in Ce0.8Y0.2O1.9 electrolyte system. J of Alloys and Compounds 509:8584–8589. https://doi.org/10.1016/j.jallcom.2011.06.036
BifaJi CT, Wang C, Tong W, JinsongXie ML (2015) Preparation and characterization of Ce0.8Y0.2–xCuxO2–δ as electrolyte for intermediate temperature solid oxide fuel cells. J Power Sources 278:420–429. https://doi.org/10.1016/j.jpowsour.2014.12.073
Dudek M, Bogusz W, Zych L, Trybalska B (2008) Electrical and mechanical properties of CeO2-based electrolytes in the CeO2–Sm2O3–M2O3 (M = La, Y) system. Solid State Ionics 179:164–167. https://doi.org/10.1016/j.ssi.2007.12.023
Dudek M, Mroz M, Zych L, D-Ciesla E (2008) Synthesis of ceria-based nano powders suitable for manufacturing solid oxide fuel cells. Materials Science-Poland 26(2):319–330. 10.11498.7080
Dudek M (2008) Ceramic oxide electrolytes based on CeO2—preparation, properties and possibility of application to electrochemical devices. J European Ceramic Society 28:965–971. https://doi.org/10.1016/j.jeurceramsoc.2007.09.004
Cheng J, Jiang Q, He H, Yang J, Wang Y, Gao J (2011) Preparation and characterization of Y2O3–Sm2O3 co-doped ceria electrolyte for IT-SOFCs. Mater Chem Phys 125:704–708. https://doi.org/10.1016/j.matchemphys.2010.09.070
Sha X, Lu Z, Haung X, Miao J, Liu Z, Xin X, Zhang Y, Su W (2007) Influence of the sintering temperature on electrical property of the Ce0.8Sm0.1Y0.1O1.9 electrolyte. J. Alloys and Compounds 433:274–278. https://doi.org/10.1016/j.jallcom.2006.06.062
Oghbaei M, Mirzaee O (2010) Microwave versus conventional sintering: a review of fundamentals, advantages and applications. J. Alloys and Compounds 494:175–189. https://doi.org/10.1016/j.jallcom.2010.01.068
Chockalingam R, Chockalingam S, Vasantha RW, Amarakoon (2011) The electrical properties of microwave sintered gadolinia doped ceria-alumina nano-composite electrolyte. J Power Sources 196:1808–1817. https://doi.org/10.1016/j.jpowsour.2010.09.074
P-Gonjal J, Heuguet R, M-Gil D, R-Calzada A, Marinel S, Moran E, Schmidt R (2015) Microwave synthesis & sintering of Sm and Ca co-doped ceria ceramics. Int J Hydrog Energy 40:15640–15651. https://doi.org/10.1016/j.ijhydene.2015.07.161
Prekajski M, Stojmenovic M, Radojkovic A, Brankovic G, Oraon H, Subasri R, Matovic B (2014) Sintering and electrical properties of Ce1−xBixO2–δ solid solution. J Alloys and Compounds 617:563–568. https://doi.org/10.1016/j.jallcom.2014.08.090
Shannon RP, Prewitt T (1969) Effective ionic radii in oxides and fluorides. Acta Crystallogr B25:925–946. https://doi.org/10.1107/S0567740869003220
Dikmen S, Aslanbay H, Dikmen E, Sahin O (2010) Hydrothermal preparation and electrochemical properties of Gd3+ and Bi3+, Sm3+, La3+, and Nd3+ codoped ceria-based electrolytes for intermediate temperature-solid oxide fuel cell. J Power Sources 195:2488–2495. https://doi.org/10.1016/j.jpowsour.2009.11.077
SubrataKundu NS, Thangamuthu R, Subramanian B, Panda AB, Jayachandran M (2012) Fabrication of catalytically active nanocrystalline samarium (Sm)-doped cerium oxide (CeO2) thin films using electron beam evaporation. J Nano part Res 14:1040. https://doi.org/10.1007/s11051-012-1040-0
Arumugam A, Karthikeyan C, Hameed ASH, Gopinath K, Gowri S, karthika V (2015) Synthesis of cerium oxide nanoparticles using Gloriosa superba L. leaf extract and their structural, optical and antibacterial properties. Mater Sci Eng C 49:408–415. https://doi.org/10.1016/j.msec.2015.01.042
Bhabu KA, Theerthagiri J, Madhavan J, Balu T, Muralidharan G, Rajasekaran TR (2016) Cubic fluorite phase of samarium doped cerium oxide (CeO2)0.96Sm0.04 for solid oxide fuel cell electrolyte. J Mater Sci Mater Electron 27:1566–1573. https://doi.org/10.1007/s10854-015-3925-z
Uslu I, ArdaAytimur M k, Serhatkocyigit (2012) Synthesis and characterization of neodymium doped ceria nanocrystalline ceramic structures. Ceram Int 38:4943–4951. https://doi.org/10.1016/j.ceramint.2012.02.087
Wattanathana W, Nootsuwan N, Veranitisagul C, Koonsaeng N, Laosiripojana N, Laobuthee A (2015) Simple cerium-triethanolamine complex: synthesis, characterization, thermal decomposition and its application to prepare ceria support for platinum catalysts used in methane steam reforming. J Mol Struct 1089:9–15. https://doi.org/10.1016/j.molstruc.2015.02.010
Limmer W, Ritter W, Sauer R (1998) Raman scattering in ion-implanted GaN. Appl Phy Lett 72:2589–2591. https://doi.org/10.1063/1.121426
Mandal BP, Roy M, Grover V, Tyagi AK (2008) X-ray diffraction, μ-Raman spectroscopic studies on CeO2–RE2O3 (RE = Ho, Er) systems: observation of parasitic phases. J. Appl. Phys 103:33506-1–33506-7. https://doi.org/10.1063/1.2837042
López JM, Gilbank AL, García T, Solsona B, Agouram S, Torrente-Murciano L (2015) The prevalence of surface oxygen vacancies over the mobility of bulk oxygen nanostuructured ceria for the total toluene oxidation. Appl Catal B Environ 174–175:403–412. https://doi.org/10.1016/j.apcatb.2015.03.017
Li S-P, Lu J-Q, Fang P, Luo M-F (2009) Effect of oxygen vacancies on electrical properties of Ce0.8Sm0.1Nd0.1O2–δ electrolyte: an in situ Raman spectroscopic study. J Power Sources 193:93–98. https://doi.org/10.1016/j.jpowsour.2008.12.022
Prashanth Kumar V, Reddy YS (2008) Thermal and electrical properties of rare-earth co-doped ceria ceramics. Mater Chem Phys 112:711–718. https://doi.org/10.1016/j.matchemphys.2008.06.030
Tanwar K, Jaiswal N, Kumar D, Parkash O (2016) Synthesis & characterization of Dy and Ca co-doped ceria based solid electrolytes for IT-SOFCs. J. Alloys and Compounds 684:683–690. https://doi.org/10.1016/j.jallcom.2016.05.223
Anderson DA, Simak SI, Skorodumova NV, Abrikosov IA, Johansson B (2006) Optimization of ionic conductivity in doped ceria. PANS 103:3518–3521. https://doi.org/10.1073/pnas.0509537103
Venkataramana K, Madhuri C, Suresh Reddy Y, Bhikshamaiah G, Vishnuvardhan Reddy C (2017) Structural, electrical and thermal expansion studies of tri-doped ceria electrolyte materials for IT-SOFCs. J. Alloys Compd 719:97–107. https://doi.org/10.1016/j.jalcom.2017.05.022
Ramesh S, Kumar VP, Kistaiah P, Reddy CV (2010) Preparation, characterization and thermo electrical properties of co-doped Ce0.8–xSm0.2CaxO2–δ materials. Solid State Ionics 181:86–91. https://doi.org/10.1016/j.ssi.2009.11.014
Wu Y-C, Lin C-C (2014) The microstructures and property analysis of aliovalent cations (Sm3+, Mg2+, Ca2+, Sr2+, Ba2+) co-doped ceria-based electrolytes after aging treatment. Int J Hydrog Energy 39:7988–8001. https://doi.org/10.1016/j.ijhydene.2014.03.063
Jin C, Yang Z, Zhang H, Yang C, Chen F (2012) La0.6Sr1.4MnO4 layered perovskite anode material for intermediate temperature solid oxide fuel cells. Electrochemistry Commun 14:75–77. https://doi.org/10.1016/j.elecom.2011.11.008
VaibhavVibhu AR, Nicollet C, AurelienFlura J-CG, Bassat J-M (2015) La2–xPrxNiO4+δ as suitable cathodes for metal supported SOFCs. Solid State Ionics 278:32–37. https://doi.org/10.1016/j.ssi.2015.05.005
Zhang L, Mo L, Huang J, Song Z (2014) Improved thermal expansion and electrochemical performances of Ba0.6Sr0.4Co0.9Nb0.1O3−δ–Gd0.1Ce0.9O1.95 composite cathodes for IT-SOFCs. Int J Hydrog Energy 39:7972–7979. https://doi.org/10.1016/j.ijhydene.2014.03.055
Acknowledgements
One of the authors, K.V., thanks the University Grants Commission (UGC), New Delhi, India, for the financial assistance under the scheme of UGC-UPE-FAR program.
Author information
Authors and Affiliations
Corresponding author
Additional information
Highlights
• The system Ce0.8Sm0.2−xYxO2−δ has been synthesized via sol–gel auto-combustion method followed by microwave sintering.
• The comparison of microwave and conventional sintering has been made.
• Influence of oxygen vacancy concentration on total ionic conductivity is investigated.
• Thermal expansion coefficient of Ce0.8Sm0.1Y0.1O2−δ is studied and compared.
• Highest total ionic conductivity of microwave sintered Ce0.8Sm0.1Y0.1O2−δ sample makes it use as a potential electrolyte for IT-SOFCs.
Rights and permissions
About this article
Cite this article
Venkataramana, K., Ravindranath, K., Madhuri, C. et al. Low temperature microwave sintering of yttrium and samarium co-doped ceria solid electrolytes for IT-SOFCs. Ionics 24, 1429–1438 (2018). https://doi.org/10.1007/s11581-017-2293-5
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11581-017-2293-5