Abstract
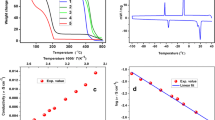
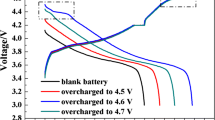
Lithium ion batteries have gained increasing attention in the last two decades. Perfoming electrolyte additive into lithium ion battery could enhance the baterry performance. However several studies on electrolyte additive only show the experimental data. The behavior of battery kinetic inside the battery and the ionic diffusion during operation was not analyzed. This study will investigate the effect of electrolyte additive on ionic diffusion of battery based on electrochemical model. The role of the electrolyte is to provide an ionic conduction path between the anode and the cathode. This study elucidates the improvement of the cycleability by performing the electrolyte containing 0.1 wt % of Flouro-o-phenylenedimaleimide (F-MI) based additive compared to the electrolyte with 0.1 wt % N,N’-o-phenylenedimaleimide (O-MI) and without an additive shown by effective constant phase elements (CPE) coefficient of impedance spectra on electrochemical impedance spectroscopy (EIS). The result elucidates that many amount of lithium ion remains on SEI layer of the mesocarbon microbeads (MCMB) half cell with F-MI additive, indicating that ion move easily because of high diffusion. The simulation result elucidated the agreement with the experimental data, the diffusion of the MCMB half-cell with F-MI additive is highest compared to MCMB half-cell with O-MI and without additive, that is emphasized with result on the EIS test.







Similar content being viewed by others
References
Hamidah NL (2013) Synthesis of fluorine functional group of maleimide based additive and its applications to the solid electrolyte interface (SEI) formation of lithium ion battery. National Taiwan University of Science and Technology, 1–155
Lee M-L, Li Y-H, Yeh J-W, Shih HC (2012) Improvement in safety and cycle life oflithium-ion batteries by employing quercetin as an electrolyte additive. J Power Sources 214:251–257
Xia L, Wang D, Yang H, Cao Y, Ai X (2012) An electrolyte additive for thermal shutdown protection of Li-ion batteries. Electrochem Commun. 25:98–100
Chan CK, Peng H, Liu G, McIlwrath K, Zhang XF, Huggins RA, Cui Y (2008) High performance lithium battery anodes using silicon nano wires. Nat Nanotechnol 3:31–35
Kasavajjula U, Wang C, Appleby AJ (2007) Nano- and bulk-silicon-based insertion anodesfor lithium-ion secondary cells. J Power Sources 163:1003–1039
Xu W, Vegunta SSS, Flake JC (2011) Surface-modified silicon nanowire anodes forlithium-ion batteries. J Power Sources 196:8583–8589
Yu H, Zhou H (2013) High-Energy Cathode Materials (Li2MnO3--LiMO2) for Lithium-IonBatteries. J Phys Chem Lett 4:1268–1280
Xu B, Qian D, Wang Z, Meng YS (2012) Recent progress in cathode materials research foradvanced lithium ion batteries. Mater Sci Eng R Rep 73:51–65
Borgel V, Markevich E, Aurbach D, Semrau G, Schmidt M (2009) On the application ofionic liquids for rechargeable Li batteries: High voltage systems. J Power Sources 189:331–336
Croce F, D_Epifanio A, Hassoun J, Reale P, Scrosati B (2003) Advanced electrolyte and electrode materials for lithium polymer batteries. J Power Sources. 119:399–402
Fan L-Z, Wang X-L, Long F (2009) All-solid-state polymer electrolyte with plastic crystalmaterials for rechargeable lithium-ion battery. J Power Sources 189:775–778
Jones J, Anouti M, Caillon-Caravanier M, Willmann P, Sizaret P-Y, Lemordant D (2011) Solubilization of SEI lithium salts in alkylcarbonate solvents. Fluid Phase Equilib 305:121–126
Jones J, Anouti M, Caillon-Caravanier M, Willmann P, Lemordant D (2010) Lithium fluoride dissolution equilibria in cyclic alkylcarbonates and water. J Mol Liq 153:146–152
Jones J, Anouti M, Caillon-Caravanier M, Willmann P, Lemordant D (2009) Thermodynamic of LiF dissolution in alkylcarbonates and some of their mixtures with water. Fluid Phase Equilib 285: 62–68
Zhang SS (2006) A review on electrolyte additives for lithium-ion batteries. J PowerSources 162:1379–1394
Wang F-M, Cheng H-M, Wu H-C, Chu S-Y, Cheng C-S, Yang C-R (2009) Novel SEI formation of maleimide-based additives and its improvement of capability and cyclicability inlithium ion batteries. Electrochim Acta 54:3344–3351
Wang F-M, Lo S-C, Cheng C-S, Chen J-H, Hwang B-J, Wu H-C (2011) Selfpolymerized membrane derivative of branched additive for internal short protection of high safety lithium ion battery. J Membr Sci 368(1–2):165–170
Kubota T, Ihara M, Katayama S, Nakai H, Ichikawa J (2012) 1,1-Difluoro-1-alkenes as newelectrolyte additives for lithium ion batteries. J Power Sources 207:141–149
Shim E-G, Nam T-H, Kim J-G, Kim H-S, Moon S-I (2007) Electrochemical performance of lithium ion batteries with triphenylphosphate as a flame-retardant additive. J Power Sources 172:919–924
He H, Xiong R, Fan J (2011) Evaluation of Lithium-Ion Battery Equivalent Circuit Models for State of Charge Estimation by an Experimental Approach. Energies 4:582–598
Lee S, Kim J, Lee J, Cho BH (2008) State-of-charge and capacity estimation of lithium-ionbattery using a new open-circuit voltage versus state-of-charge. J Power Sources 185:1367–1373
Xiong R, He H, Guo H, Ding Y (2011) Modeling for Lithium-Ion Battery used in Electric Vehicles. Procedia Eng 15:2869–2874
Wang F, Yu M, Hsiao Y, Tsai Y, Hwang B, Wang Y (2011) Aging Effects to Solid Electrolyte Interface (SEI) Membrane Formation and the Performance Analysis of Lithium Ion Batteries. Int J Electrochem Sci 6:1014–1026
Wang C, Appleby AJ, Little FE (2001) Electrochemical impedance study of initial lithium ion intercalation into graphite powders. Electrochim Acta 46:1793–1813
Besenhard JO, Winter M, Yang J, Biberacher W (1995) Filming mechanism of lithium-carbon anodes in organic and inorganic electrolytes. J Power Sources 54:228–231
Shim E-G, Nam T-H, Kim J-G, Kim H-S, Moon S-I (2007) Electrochemical performance of lithium-ion batteries with triphenylphosphate as a flame-retardant additive. J Power Sources 172:919–924
Acknowledgments
The authors are grateful for financially supporting this research from the Directorate General of Higher Education, The Ministry of Education and Culture Republic of Indonesia (contract no. 038839.36/IT2.11/PN.01.00/2014). The technical assistance of the Sustainable Energy Division, National Taiwan University of Science and Technology (NTUST), under Prof. Fu Ming Wang’s guidance is greatly appreciated.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Hamidah, N.L., Nugroho, G. & Wang, F.M. Electrochemical analysis of electrolyte additive effect on ionic diffusion for high-performance lithium ion battery. Ionics 22, 33–41 (2016). https://doi.org/10.1007/s11581-015-1505-0
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11581-015-1505-0