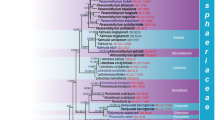
Abstract
A new genus Rhopalophora is described for Phialophora clavispora, a lignicolous species formerly placed in Phialophora section Catenulatae that possesses pigmented conidiophores, phialides with a single conidiogenous locus that occasionally appear as schizophialides, and clavate, aseptate conidia arranged in chains or sometimes in heads. Sexual morphs are not known for this taxon. Phylogenetic analysis of DNA sequences from five loci (nucSSU, ITS, nucLSU, mitSSU, rpb1 and rpb2) of this and related fungi supports the introduction of a new family, Sclerococcaceae, for which we establish the order Sclerococcales. This order belongs to the new subclass Sclerococcomycetidae, a strongly supported clade within the Eurotiomycetes that is basal to a lineage containing the Chaetothyriomycetidae, Coryneliomycetidae and Eurotiomycetidae. Rhopalophora clavispora fits in this new family and is closely related to an isolate of Fusichalara minuta. The Sclerococcales also encompass marine, lignicolous species of Dactylospora, two species of the lichenicolous genus Sclerococcum, and a lineage comprised of strains from the digestive tracts of Neotropical wood-inhabiting beetles. We confirm that Dactylospora is polyphyletic; the phylogenetic placement of D. parasitica, the generic type, remains unknown.







Similar content being viewed by others
References
Bellemère A, Hafellner J (1982) L’ultrastructure des asques de genre Dactylospora (Discomycetes) et son interet taxonomique. Cryptogam Mycol 3:71–93
Bhat DJ, Kendrick WB (1993) Twenty-five new conidial fungi from the western Ghats and the Andaman islands (India). Mycotaxon 49:19–90
Bogale M, Orr MJ, O’Hara MJ, Untereiner WA (2010) Systematics of Catenulifera (anamorphic Hyaloscyphaceae) with an assessment of the phylogenetic position of Phialophora hyalina. Fung Biol 114:396–409
Cannon PF, Kirk PM (2007) Fungal families of the world. CAB International, Wallingford
Cole GT, Kendrick WB (1973) Taxonomic studies of Phialophora. Mycologia 65:661–688
Constantinescu O, Holm K, Holm J (1995) Teleomorph-anamorph connections in Ascomycetes: the anamorphs of three species of Chaetosphaeria. Mycol Res 99:585–592
Crittenden PD, David JC, Hawksworth DL, Campbell FS (1995) Attempted isolation and success in the culturing of a broad spectrum of lichen-forming and lichenicolous fungi. New Phytol 130:267–297
Crous PE, Schubert K, Braun U, de Hoog GS, Hocking AD, Shin HD, Groenewald JZ (2007) Opportunistic, human-pathogenic species in the Herpotrichiellaceae are phenotypically similar to saprobic or phytopathogenic species in the Venturiaceae. Stud Mycol 58:185–217
De Beer ZW, Seifert KA, Wingfield MJ (2013) A nomenclator for ophiostomatoid genera and species in the Ophiostomatales and Microascales. CBS Biodiver Ser 12:245–322
De Hoog GS, Weenink XO, Gerrits van den Ende AH (1999) Taxonomy of the Phialophora verrucosa complex with the description of two new species. Stud Mycol 43:107–122
De Hoog GS, Guarro J, Gené J, Figueras MJ (2000) Atlas of clinical fungi 2nd edn. Centraalbureau voor Schimmelcultures, Utrecht and Universitat Rovira I Virgili, Reus
De Hoog GS, Mayser P, Haase G, Horre R, Horrevorts AM (2000b) A new species, Phialophora europaea, causing superficial infections in humans. Mycoses 43:409–416
Diederich P, Ertz D, Lawrey JD, Sikaroodi M, Untereiner WA (2013) Molecular data place the hyphomycetous lichenicolous genus Sclerococcum close to Dactylospora (Eurotiomycetes) and S. parmeliae in Cladophialophora (Chaetothyriales). Fungal Divers 58:61–72
Gams W (1971) Cephalosporium-artige Schimmelpilze. Gustav Fischer Verlag, Stuttgart
Gams W (2000) Phialophora and some similar morphologically little-differentiated anamorphs of divergent ascomycetes. Stud Mycol 45:187–200
Gams W, Holubová-Jechová V (1976) Chloridium and some other Dematiaceous Hyphomycetes growing on decaying wood. Stud Mycol 13:1–99
Gams W, Hoekstra ES, Aptroot A (Eds.) (1998) CBS Course of Mycology, Centraalbureau voor Schimmelcultures, Baarn
Gargas A, Taylor JW (1992) Polymerase chain reaction (PCR) primers for amplifying and sequencing nuclear 18S rDNA from lichenized fungi. Mycologia 84:589–592
Geiser DM, Gueidan C, Miadlikowska J, Lutzoni F, Kauff F, Hofstetter V, Fraker E, Schoch CL, Tibell L, Untereiner WA, Aptroot A (2007) Eurotiomycetes: eurotiomycetidae and Chaetothyriomycetidae. Mycologia 98:1053–1064
Gueidan C, Roux C, Lutzoni F (2007) Using a multigene phylogenetic analysis to assess generic delineation and character evolution in Verrucariaceae (Eurotiomycetes, Ascomycota). Mycol Res 111:1145–1168
Gueidan C, Ruibal VC, de Hoog GS, Gorbushina AA, Untereiner WA, Lutzoni F (2008) An extremotolerant rock-inhabiting ancestor for mutualistic and pathogen-rich fungal lineages. Stud Mycol 61:111–119
Gueidan C, Aptroot A, da Silva Cáceres ME, Badali H, Stenroos S (2014) A reappraisal of orders and families within the subclass Chaetothyriomycetidae (Eurotiomycetes, Ascomycota). Mycol Prog 13:1027–1039
Gutell RR (1993) Collection of small subunit (16S- and 16S-like) ribosomal RNA structures. Nucleic Acids Res 21:3051–3054
Gutell RR, Gray MW, Schnare MN (1993) A compilation of large subunit (23S and 23S-like) ribosomal RNA structures. Nucleic Acids Res 21:3055–3074
Haase G, Sonntag L, Melzer-Krick B, de Hoog GS (1999) Phylogeny inference by SSU gene analysis of members of the Herpotrichiellaceae with special reference to human pathogenic species. Stud Mycol 43:80–97
Hafellner J (1979) Karschia. Revision einer Sammelgattung an der Grenze von lichenisierten und nichtlichenisierten Ascomyceten. Beih Nova Hedw 62:1–248
Hall TA (1999) BioEdit 5.0.9: a user-friendly biological sequence alignment editor and analysis program for Windows 95/98/NT. Nucleic Acids Symp Ser 41:95–98
Hausner G, Reid J, Klassen GR (1993) On the subdivision of Ceratocystis s.l., based on partial ribosomal DNA sequences. Can J Bot 71:52–63
Hawksworth DL (1975) Notes on British lichenicolous fungi, I. Kew Bull 30:183–203
Hawksworth DL (1979) The lichenicolous Hyphomycetes. Bull Br Mus Nat Hist Botany 6:183–300
Hawksworth DL, Jones D (1981) Sclerococcum sphaerale obtained in pure culture. Trans Br Mycol Soc 77:485–489
Hofstetter V, Miadlikowska J, Kauff F, Lutzoni F (2007) Phylogenetic comparison of protein-coding versus ribosomal RNA coding sequence data: a case study of the Lecanoromycetes (Ascomycota). Mol Phylogenet Evol 44:412–426
Hosoya T (2002) Hyaloscyphaceae in Japan (6): The genus Hyphodiscus in Japan and its anamorph Catenulifera gen. nov. Mycoscience 43:47–57
Huelsenbeck JP, Ronquist F (2001) MrBayes: Bayesian inference of phylogenetic trees. Bioinformatics 17:754–755
Hughes SJ (1951) Studies on microfungi. XI. Some hyphomycetes which produce phialides. Mycol Pap 45:1–36
Hughes SJ, Nag Raj TR (1973) New Zealand fungi. 20. Fusichalara gen. nov. New Zeal J Bot 11:661–671
Ihlen PG, Holien H, Tønsberg T (2004) Two new species of Dactylospora (Dactylosporaceae, Lecanorales), with a key to the species in Scandinavia. Bryologist 107:357–362
Iwatsu T, Miyaji M (1977) Subcutaneous cystic granuloma caused by Phialophora verrucosa. Mycopathologia 64:165–168
Johnston P, Seifert KA, Stone J, Rossman AY, Marvanová L (2014) Recommendations on generic names competing for use in Leotiomycetes (Ascomycota). IMA Fungus 5:91–120
Jones EBG, Abdel-Wahab MA, Alias SA, Hsieh SY (1999) Dactylospora mangrovei sp. nov. (Discomycetes, ascomycota) from mangrove wood. Mycoscience 40:317–320
Katoh K, Standley DM (2013) MAFFT multiple sequence alignment software version 7: improvements in performance and usability. Mol Biol Evol 30:772–780
Kauff F, Lutzoni F (2002) Phylogeny of the Gyalectales and Ostropales (Ascomycota, Fungi): among and within order relationships based on nuclear ribosomal RNA small and large subunits. Mol Phylogenet Evol 25:138–156
Kirk PM, Spooner BM (1984) An account of the fungi of Arran, Gigha and Kintyre. Kew Bull 38:503–597
Kohlmeyer J (1967) Intertidal and phycophilous fungi from Tenerife (Canary Islands). Trans Brit Mycol Soc 50:137–147
Kohlmeyer J, Kohlmeyer E (1965) New marine fungi from mangroves and trees along eroding shorelines. Nova Hedwigia 9:89–104
Kohlmeyer J, Kohlmeyer E (1971) Marine fungi from tropical America and Africa. Mycologia 63:831–861
Kohlmeyer J, Volkmann-Kohlmeyer B (1998) Dactylospora canariensis sp. nov. and notes on D. haliotrepha. Mycotaxon 67:247–250
Kornerup A, Wanscher JH (1978) Methuen handbook of colour, 3rd edn. Eyre Methuen, London
Koukol O (2016) Myriococcum revisited: a revision of an overlooked fungal genus. Plant Syst Evol. doi:10.1007/s00606-016-1310-x
Landvik S (1996) Neolecta, a fruit-body-producing genus of the basal ascomycetes, as shown by SSU and LSU DNA sequences. Mycol Res 100:199–202
Li Y, Xiao J, de Hoog GS, Wang X, Wan Z, Yu J, Liu W, Li R (2016) Biodiversity and human-pathogenicity of Phialophora verrucosa and relatives in Chaetothyriales. Persoonia 38:1−19
Liu YJ, Whelen S, Hall BD (1999) Phylogenetic relationships among ascomycetes: evidence from an RNA polymerase II subunit. Mol Biol Evol 16:1799–1808
Lumbsch HT, Huhndorf SM (2010) Myconet volume 14. Part One. Outline of Ascomycota—2009. Part Two. Notes on Ascomycete Systematics. Nos. 4751–5113. Fieldiana Life Earth Sci 1:1–64
Malloch D (1981) Moulds: their isolation, cultivation and identification. University of Toronto Press, Toronto
Malloch D, Cain RF (1970) Five new genera in the new family Pseudoeurotiaceae. Can J Bot 48:1815–1825
Mason-Gamer RJ, Kellogg EA (1996) Testing for phylogenetic conflict among molecular data sets in the tribe Triticeae (Gramineae). Syst Biol 45:524–545
Medlar JM (1915) A new fungus, Phialophora verrucosa, pathogenic for man. Mycologia 7:200–203
Miadlikowska J, Kauff F, Högnabba F, Oliver JC, Molnár K, Fraker E, Gaya E, Hafellner J, Hofstetter V, Gueidan C, Otálora MAG, Hodkinson B, Kukwa M, Lücking R, Björk C, Sipman HJM, Burgaz AR, Thell A, Passo A, Myllys L, Goward T, Fernández-Brime S, Hestmark G, Lendemer J, Lumbsch HT, Schmull M, Schoch C, Sérusiaux E, Maddison DR, Arnold AE, Lutzoni F, Stenroos S (2014) A multigene phylogenetic synthesis for the class Lecanoromycetes (Ascomycota): 1307 fungi representing 1139 infrageneric taxa, 317 genera and 66 families. Mol Phyl Evol 79:132–68
Miller AN, Huhndorf SM (2004) A natural classification of Lasiosphaeria based on nuclear LSU rDNA sequences. Mycol Res 108:26–34
Müller E, Petrini O, Fisher PJ, Samuels GJ, Rossman AY (1987) Taxonomy and anamorphs of the Herpotrichiellaceae with notes on generic synonymy. Trans Br Mycol Soc 88:63–74
Nag Raj TR, Kendrick B (1975) A monograph of Chalara and allied genera. Wilfred Laurier, Waterloo
Nylander J (2008) MrModeltest2 v. 2.3 (Program for selecting DNA substitution models using PAUP*). Evolutionary Biology Centre, Uppsala, Sweden
Pang KL, Guo SY, Alas SA, Hafellner J, Jones EBG (2014) A new species of marine Dactylospora and its phylogenetic affinities within the Eurotiomycetes, Ascomycota. Bot Mar 57:315–321
Petrini LE, Petrini O, Candoussau F (1984) Anamorphs of Ascmycetes: the Phialophora-like state of Eosphaeria uliginosa (Syn. Herminia dichroospora). Trans Br Mycol Soc 82:554–556
Réblová M (2004) Four new species of Chaetosphaeria from New Zealand and redescription of Dictyochaeta fuegiana. Stud Mycol 50:171–186
Réblová M, Gams W, Štěpánek V (2011) The new hyphomycete genera Brachyalara and Infundichalara, the similar Exochalara and species of ‘Phialophora sect. Catenulatae’ (Leotiomycetes). Fung Diver 46:67–87
Réblová M, Untereiner W, Réblová K (2013) Novel evolutionary lineages revealed in the Chaetothyriales (Fungi) based on multigene phylogenetic analyses and comparison of ITS secondary structure. PLoS ONE 8(5), e63547
Réblová M, Miller AN, Rossman AY, Seifert KA, Crous PW, Hawksworth DL, Abdel-Wahab MA, Cannon PF, Daranagama DA, de Beer ZW, Huang SK, Hyde KD, Jayawardena R, Jaklitsch W, Jones EBG, Ju YM, Judith C, Maharachchikumbura SSN, Pang KL, Petrini LE, Raja HA, Romero AI, Shearer C, Senanayake IC, Voglmayr H, Weir BS, Wijayawarden NN (2016) Recommendations for competing sexual-asexually typified generic names in Sordariomycetes (except Diaporthales, Hypocreales, and Magnaporthales). IMA Fungus 7:131–153
="4"/>Reeb V, Lutzoni F, Roux C (2004) Contribution of RPB2 to multilocus phylogenetic studies of the euascomycetes (Pezizomycotina, Fungi) with special emphasis on the lichen-forming Acarosporaceae and evolution of polyspory. Mol Phylogenet Evol 32:1036–1060
Rehner SA, Samuels GJ (1994) Taxonomy and phylogeny of Gliocladium analysed from nuclear large subunit ribosomal DNA sequences. Mycol Res 98:625–634
Rossman AY, Schoch CL, Farr DF, Nishijima K, Keith L, Goenaga R (2010) Dolabra nepheliae on rambutan and lychee represents a novel lineage of phytopathogenic Eurotiomycetes. Mycoscience 51:300–309
Sarrión FJ, Hafellner J, Burgaz AR (2002) Three new species of the genus Dactylospora in Spain. Lichenologist 34:361–368
Schoch C, Sung GH, López-Giráldez F, Townsend JP, Miadlikowska J, Hofstetter V, Robbertse B, Matheny B, Kauff F, Wang Z, Gueidan C, Andrie RM, Trippe K, Ciufetti LM, Wynns A, Fraker E, Hodkinson BP, Bonito G, Groenewald JZ, Arzanlou M, De Hoog GS, Crous PW, Hewitt D, Pfister D, Peterson K, Gryzenhout M, Wingfield MJ, Aptroot A, Suh SO, Blackwell M, Hillis DM, Griffith GW, Castlebury LA, Rossman A, Lumbsch HT, Lücking R, Büdel B, Rauhut A, Diederich P, Ertz D, Geiser DM, Hosaka K, Inderbitzin P, Kohlmeyer J, Volkmann-Kohlmeyer B, Mostert L, O’Donnell K, Sipman H, Rogers JD, Shoemaker R, Sugiyama J, Summerbell RC, Untereiner W, Johnston PR, Stenroos S, Zuccaro A, Dyer PS, Crittenden PD, Cole MS, Hansen K, Trappe JM, Yahr R, Lutzoni F, Spatafora JW (2009) The Ascomycota tree of life: a phylum-wide phylogeny clarifies the origin and evolution of fundamental reproductive and ecological traits. Syst Biol 58:224–239
Schol-Schwarz MB (1970) Revision of the genus Phialophora (Moniliales). Persoonia 6:59–94
Sivasithamparam K (1975) Phialophora and Phialophora-like fungi occurring in the root region of wheat. Aust J Bot 23:157–164
Spatafora JW, Mitchell TG, Vilgalys R (1995) Analysis of genes coding for small-subunit rRNA sequences in studying phylogenetics of dematiaceous fungal pathogens. Eur J Clin Microbiol 33:1322–1326
Spatafora JW, Johnson D, Sung G-H, Hosaka K, O’Rourke B, Serdani M, Spotts R, Lutzoni F, Hofstetter V, Fraker E, Gueidan C, Miadlikowska J, Reeb V, Lumbsch T, Lücking R, Schmitt I, Aptroot A, Roux C, Miller A, Geiser D, Hafellner J, Hestmark G, Arnold AE, Büdel B, Rauhut A, Hewitt D, Untereiner W, Cole MS, Scheidegger C, Schultz M, Sipman H, Schoch CL (2007) A five-gene phylogenetic analysis of the Pezizomycotina. Mycologia 98:1020–1030
Stamatakis A (2006) RAxML-VI-HPC: maximum likelihood-based phylogenetic analyses with thousands of taxa and mixed models. Bioinformatics 22:2688–2690
Stenroos S, Laukka T, Huhtinen S, Döbbeler P, Myllys L, Syrjänen K, Hyvönen J (2010) Multiple origins of symbioses between ascomycetes and bryophytes suggested by a five-gene phylogeny. Cladistics 26:281–300
Untereiner WA (1995) Fruiting studies in species of Capronia (Herpotrichiellaceae). Antonie Van Leeuwenhoek 68:3–17
Untereiner WA (2000) Capronia and its anamorphs: exploring the value of morphological and molecular characters in the systematics of the Herpotrichiellaceae. Stud Mycol 45:141–148
Untereiner WA, Naveau FA (1999) Molecular systematics of the Herpotrichiellaceae with an assessment of the phylogenetic positions of Exophiala dermatitidis and Phialophora americana. Mycologia 91:67–83
Untereiner WA, Straus NA, Malloch D (1995) A molecular-morphotaxonomic approach to the systematics of the Herpotrichiellaceae and allied black yeasts. Mycol Res 99:897–913
Untereiner WA, Naveau FA, Bachewich J, Angus A (2006) Evolutionary relationships of Hyphodiscus hymeniophilus (anamorph Catenulifera rhodogena) inferred from β-tubulin and nuclear ribosomal DNA sequences. Can J Bot 84:243–253
Untereiner WA, Angus A, Réblová M, Orr MJ (2008) Systematics of the Phialophora verrucosa complex: new insights from analyses of β-tubulin, large subunit nuclear rDNA and ITS sequences. Botany 86:742–750
Vargas-Asensio G, Pinto-Tomas A, Rivera B, Hernandez M, Hernandez C, Soto-Montero S, Murillo C, Sherman DH, Tamayo-Castillo G (2014) Uncovering the cultivable microbial diversity of Costa Rican beetles and its ability to break down plant cell wall components. PLoS ONE 9(11), e113303
Vilgalys R, Hester M (1990) Rapid genetic identification and mapping of enzymatically amplified ribosomal DNA from several Cryptococcus species. J Bacteriol 172:4238–4246
White TJ, Bruns T, Lee S, Taylor J (1990) Amplification and direct sequencing of fungal ribosomal RNA genes for phylogenetics. In: Innis MA, Gelfand DH, Sninsky JJ, White TJ (eds) PCR protocols: a guide to methods and applications. Academic, San Diego, pp 315–322
Wood AR, Damm U, van der Linde EJ, Groenewald JZ, Cheewangkoon R, Crous PW (2016) Finding the missing link: Resolving the Coryneliomycetidae within Eurotiomycetes. Persoonia 37:37–56
Zoller S, Scheidegger C, Sperisen C (1999) PCR primers for the amplification of mitochondrial small subunit ribosomal DNA of lichen-forming ascomycetes. Lichenologist 31:511–516
Acknowledgements
This study was supported by the Project of the National Foundation of the Czech Republic (GACR 506/12/0038, www.gacr.cz), and as a long-term research development project of the Institute of Botany, Academy of Sciences (No. RVO 67985939) and the Institute of Microbiology, Academy of Sciences (No. RVO 61388971). Funding to WAU was in the form of a Discovery Grant from the Natural Science and Engineering Research Council of Canada (www.nserc-crsng.gc.ca) and infrastructure awards from the Canada Foundation for Innovation. We are grateful to Johannes Z. (Ewald) Groenewald for providing access to the unpublished ITS sequence data in the CBS-KNAW Fungal Biodiversity Centre culture collection database. We thank Gerard Verkleij for his assistance with obtaining strains of Phialophora clavispora and Fusichalara minuta. We are grateful to Zdeněk Palice for a loan of his herbarium material of Dactylospora parasitica.
Author information
Authors and Affiliations
Corresponding author
Additional information
Section Editor: Roland Kirschner and Pedro W. Crous
Rights and permissions
About this article
Cite this article
Réblová, M., Untereiner, W.A., Štěpánek, V. et al. Disentangling Phialophora section Catenulatae: disposition of taxa with pigmented conidiophores and recognition of a new subclass, Sclerococcomycetidae (Eurotiomycetes). Mycol Progress 16, 27–46 (2017). https://doi.org/10.1007/s11557-016-1248-y
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11557-016-1248-y