Abstract
Purpose
The objective of this study was to develop and validate a novel prognostic nomogram to evaluate the survival benefit of hepatocellular carcinoma (HCC) patients receiving postoperative adjuvant transarterial chemoembolization (PA-TACE).
Materials and methods
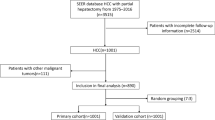
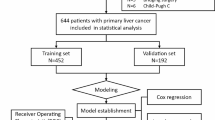
Clinical data of HCC patients who underwent hepatectomy at four medical centers were retrospectively analyzed, including those who received PA-TACE and those who did not. These two categories of patients were randomly allocated to the development and validation cohorts in a 7:3 ratio.
Results
A total of 1505 HCC patients who underwent hepatectomy were included in this study, comprising 723 patients who did not receive PA-TACE and 782 patients who received PA-TACE. Among them, patients who received PA-TACE experienced more adverse events, although these events were mild and manageable (Grade 1–2, all p < 0.05). Nomograms were constructed and validated for patients with and without PA-TACE using independent predictors that influenced disease-free survival (DFS) and overall survival (OS). These two nomograms had C-indices greater than 0.800 in the development cohort and exhibited good calibration and discrimination ability compared to six conventional HCC staging systems. Patients in the intermediate-to-high-risk group in the nomogram who received PA-TACE had higher DFS and OS (all p < 0.05). In addition, tumor recurrence was significantly controlled in the intermediate-to-high-risk group of patients who received PA-TACE, while there was no significant difference in the low-risk group of patients who received PA-TACE.
Conclusion
The nomograms were developed and validated based on large-scale clinical data and can serve as online decision-making tools to predict survival benefits from PA-TACE in different subgroups of patients.





Similar content being viewed by others
Data availability
Data will be made available on reasonable request due to restrictions, e.g., privacy or ethical.
Abbreviations
- HCC:
-
Hepatocellular carcinoma
- PA-TACE:
-
Postoperative adjuvant transcatheter chemoembolization
- EASL:
-
European Association for the study of the liver
- AASLD:
-
American Association for the study of liver diseases
- APASL:
-
Asian Pacific Association for the study of the liver
- AFP:
-
Alpha-fetoprotein
- ALT:
-
Alanine aminotransferase
- AST:
-
Aspartate aminotransferase
- GGT:
-
Gamma-glutamyltransferase
- ALP:
-
Alkaline phosphatase
- ALB:
-
Albumin
- TB:
-
Total bilirubin
- WBC:
-
White blood cell
- CR:
-
Creatinine
- PT:
-
Prothrombin time
- NLR:
-
Neutrophil-to-lymphocyte ratio
- LMR:
-
Lymphocyte-to-monocyte ratio
- PLR:
-
Platelet-to-lymphocyte ratio
- HBV:
-
Hepatitis B virus
- MVI:
-
Microvascular invasion
- CT:
-
Computed tomography
- MRI:
-
Magnetic resonance imaging
- DFS:
-
Disease-free survival
- OS:
-
Overall survival
- ROC:
-
Receiver operating characteristic
- AUC:
-
Area under the curve
- C-Index:
-
Concordance index
- HR:
-
Hazard ratio
- Cl:
-
Confidence interval
- CNLC:
-
China liver cancer
- BCLC:
-
Barcelona clinic liver cancer
- AJCC:
-
American joint committee on cancer
- TNM:
-
Tumor node metastasis
- JIS:
-
Japan integrated staging
- HKLC:
-
Hong Kong liver cancer
References
H Sung J Ferlay RL Siegel M Laversanne I Soerjomataram A Jemal F Bray 2021 Global cancer statistics 2020: GLOBOCAN estimates of incidence and mortality worldwide for CA 71 3 209 249
Erratum (2020) Global cancer statistics 2018: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA, 70(4):313
R Kloeckner PR Galle J Bruix 2021 Local and regional therapies for hepatocellular carcinoma Hepatology 73 137 149
A Vogel A Cervantes I Chau B Daniele JM Llovet T Meyer JC Nault 2018 Hepatocellular carcinoma: ESMO clinical practice guidelines for diagnosis, treatment and follow-up. Ann Oncol. 29 4 238 255
European Association for the Study of the Liver 2012 European Organisation for Research and Treatment of Cancer EASL-EORTC clinical practice guidelines: management of hepatocellular carcinoma J Hepatol 56 4 908 943
JA Marrero LM Kulik CB Sirlin AX Zhu RS Finn MM Abecassis 2018 Diagnosis, staging, and management of hepatocellular carcinoma: 2018 practice guidance by the American Association for the Study of Liver Diseases Hepatology 68 2 723 750
M Omata AL Cheng N Kokudo M Kudo JM Lee J Jia 2017 Asia-Pacific clinical practice guidelines on the management of hepatocellular carcinoma: a 2017 update Hepatol Int 11 4 317 370
L Liang C Li YK Diao HD Jia H Xing TM Pawlik WY Lau 2020 Survival benefits from adjuvant transcatheter arterial chemoembolization in patients undergoing liver resection for hepatocellular carcinoma: a systematic review and meta-analysis Ther Adv Gastroenterol 13 1756284820977693
H Wang PC Du MC Wu WM Cong 2018 Postoperative adjuvant transarterial chemoembolization for multinodular hepatocellular carcinoma within the Barcelona Clinic Liver Cancer early stage and microvascular invasion Hepatobiliary Surg Nutrit 7 6 418 428
K Wang YJ Xiang HM Yu YQ Cheng YY Qin WJ Wang XP Zhang 2022 A novel classification in predicting prognosis and guiding postoperative management after R0 liver resection for patients with hepatocellular carcinoma and microvascular invasion Eur J Surg Oncol 48 6 1348 1355
F Liu X Guo W Dong W Zhang S Wei S Zhang X Zhu 2020 Postoperative adjuvant TACE-associated nomogram for predicting the prognosis of resectable Hepatocellular Carcinoma with portal vein Tumor Thrombus after Liver Resection Int J Biol Sci 16 16 3210 3220
YF Zhang H Shang XL Zeng H Ji YM Li HW Lu 2018 Postoperative adjuvant chemo (embolization) therapy for hepatocellular carcinoma with portal vein tumor thrombosis Onco Targets Ther 11 5407 5417
YP Qi JH Zhong ZY Liang J Zhang B Chen CZ Chen LQ Li 2019 Adjuvant transarterial chemoembolization for patients with hepatocellular carcinoma involving microvascular invasion Am J Surg 217 4 739 744
L Wang Q Ke N Lin Y Zeng J Liu 2019 Does postoperative adjuvant transarterial chemoembolization benefit for all patients with hepatocellular carcinoma combined with microvascular invasion: a meta-analysis Scand J Gastroenterol 54 5 528 537
A Sugioka Y Kato Y Tanahashi 2017 Systematic extrahepatic Glissonean pedicle isolation for anatomical liver resection based on Laennec's capsule: proposal of a novel comprehensive surgical anatomy of the liver J Hepatobiliary Pancreat Sci 24 1 17 23
CE Costentin CR Ferrone RS Arellano S Ganguli TS Hong AX Zhu 2017 Hepatocellular carcinoma with macrovascular invasion: defining the optimal treatment strategy Liver cancer 6 4 360 374
YY Wang LJ Wang D Xu M Liu HW Wang K Wang X Zhu 2019 Postoperative adjuvant transcatheter arterial chemoembolization should be considered selectively in patients who have hepatocellular carcinoma with microvascular invasion HPB (Oxford) 21 4 425 433
Y Li Y Zhang Q Fang X Zhang P Hou H Wu X Wang 2021 Radiomics analysis of [F]FDG PET/CT for microvascular invasion and prognosis prediction in very-early- and early-stage hepatocellular carcinoma Eur J Nucl Med Mol Imaging 48 8 2599 2614
European Association for Study of Liver 2012 European Organisation for Research and Treatment of Cancer: EASL-EORTC clinical practice guidelines: management of hepatocellular carcinoma Eur J Cancer 48 5 599 641
National Cancer Institute: common terminology criteria for adverse events, version 5.0. 2020: https://ctep.cancer.gov/protocolDevelopment/electronic_applications/docs/CTCAE_v5_Quick_Reference_5x7.
MS Schröder AC Culhane J Quackenbush B Haibe-Kains 2011 survcomp: an R/Bioconductor package for performance assessment and comparison of survival models Bioinformatics 27 22 3206 3208
PJ Heagerty T Lumley MS Pepe 2000 Time-dependent ROC curves for censored survival data and a diagnostic marker Biometrics 56 2 337 344
ME Cohen CY Ko KY Bilimoria L Zhou K Huffman X Wang Y Liu 2013 Optimizing ACS NSQIP modeling for evaluation of surgical quality and risk: patient risk adjustment, procedure mix adjustment, shrinkage adjustment, and surgical focus J Am College Surg. 217 2 336 346
AJ Vickers EB Elkin 2006 Decision curve analysis: a novel method for evaluating prediction models Med Decis Making 26 6 565 574
RL Camp M Dolled-Filhart DL Rimm 2004 X-tile: a new bio-informatics tool for biomarker assessment and outcome-based cut-point optimization Clin Cancer Res 10 21 7252 7259
A Vogel A Cervantes I Chau B Daniele JM Llovet T Meyer JC Nault 2018 Hepatocellular carcinoma: ESMO clinical practice guidelines for diagnosis, treatment and follow-up Ann Oncol. 29 iv238 iv255
X Chen B Zhang X Yin Z Ren S Qiu J Zhou 2013 Lipiodolized transarterial chemoembolization in hepatocellular carcinoma patients after curative resection J Cancer Res Clin Oncol 139 5 773 781
Y-C Cheng T-W Chen H-L Fan C-Y Yu H-C Chang C-B Hsieh 2014 Transarterial chemoembolization for intrahepatic multiple recurrent HCC after liver resection or transplantation Ann Transplant 19 309 316
P Bhargava DC Gammon D Kim D Phillips S Mehta L Shick L Cicalese 2004 Chemoembolization (CE) with carboplatin and doxorubicin for hepatocellular cancer (HCC): introduction of a novel combination and preliminary results J Clin Oncol. 22 14_suppl 4275 4275
W Wei PE Jian SH Li ZX Guo YF Zhang YH Ling 2018 Adjuvant transcatheter arterial chemoembolization after curative resection for hepatocellular carcinoma patients with solitary tumor and microvascular invasion: a randomized clinical trial of efficacy and safety Cancer Commun (Lond) 38 1 61
Z Wang Z Ren Y Chen J Hu G Yang L Yu X Yang 2018 Adjuvant transarterial chemoembolization for HBV-related hepatocellular carcinoma after resection: a randomized controlled studyadjuvant TACE for HCC after resection Clin Cancer Res 24 9 2074 2081
J-L Raoul A Forner L Bolondi TT Cheung R Kloeckner T Baere de 2019 Updated use of TACE for hepatocellular carcinoma treatment: How and when to use it based on clinical evidence Cancer Treat Rev 72 28 36
Funding
This work was funded by Zhongshan Science and Technology Plan Project of Guangdong Province (Project Number: 2021B1040), Key research and development projects of Jiangxi Provincial Department of Science and Technology (Project Number: 20202BBGL73092), Natural Science Foundation of Jiangxi Provincial (Project Number: 20171BAB205064) and National Natural Science Foundation of China (Project Number: 81860432) that have no role in the collection, analysis, interpretation of results or writing of the manuscripts.
Author information
Authors and Affiliations
Contributions
KH and YL contributed to the study concept and design. YH, JQ, GZ, and ZW were involved in the conceptualization of methodology. YH, JQ, GZ, ZW, LC, ST, LL, and RS assisted in the acquisition of data. YH, JQ, GZ, and ZW contributed to the analysis and interpretation of data. YH, JQ, GZ, ZW, KH, and YL were involved in the drafting of the manuscript.
Corresponding authors
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Ethics approval
The study was approved by the ethics committees of the First Affiliated Hospital of Nanchang University, the Second Affiliated Hospital of Nanchang University, Shenzhen People's Hospital and Zhongshan People's Hospital, and followed the guidelines of the Declaration of Helsinki (Ethics number:2022-CDYFYYLK-08–015). Written informed consent was obtained from all patients for their data to be used for scientific purposes.All procedures involving human participants were performed in accordance with the 1975 Helsinki declaration and its lateramendments.
Informed consent
Written informed consent was obtained from all patients for their data to be used for scientific purposes.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
He, Y., Qian, J., Zhu, G. et al. Development and validation of nomograms to evaluate the survival outcome of HCC patients undergoing selective postoperative adjuvant TACE. Radiol med 129, 653–664 (2024). https://doi.org/10.1007/s11547-024-01792-0
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11547-024-01792-0