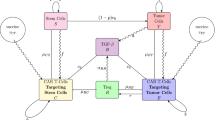
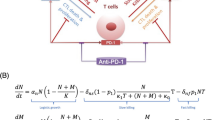
Abstract
Chimeric antigen receptor (CAR)-engineered natural killer (NK) cells have recently emerged as a promising and safe alternative to CAR-T cells for targeting solid tumors. In the case of triple-negative breast cancer (TNBC), traditional cancer treatments and common immunotherapies have shown limited effectiveness. However, CAR-NK cells have been successfully employed to target epidermal growth factor receptor (EGFR) on TNBC cells, thereby enhancing the efficacy of immunotherapy. The effectiveness of CAR-NK-based immunotherapy is influenced by various factors, including the vaccination dose, vaccination pattern, and tumor immunosuppressive factors in the microenvironment. To gain insights into the dynamics and effects of CAR-NK-based immunotherapy, we propose a computational model based on experimental data and immunological theories. This model integrates an individual-based model that describes the interplay between the tumor and the immune system, along with an ordinary differential equation model that captures the variation of inflammatory cytokines. Computational results obtained from the proposed model shed light on the conditions necessary for initiating an effective anti-tumor response. Furthermore, global sensitivity analysis highlights the issue of low persistence of CAR-NK cells in vivo, which poses a significant challenge for the successful clinical application of these cells. Leveraging the model, we identify the optimal vaccination time, vaccination dose, and time interval between injections for maximizing therapeutic outcomes.














Similar content being viewed by others
Data Availability
The data that support the findings of this study are available from the corresponding author, upon reasonable request.
Code Availability
The MATLAB code files implementing the model described in this study is available from the corresponding author, upon reasonable request.
References
Altrock PM, Kimmel G, Locke FL (2018) Evolutionary dynamics of non-Hodgkin's lymphoma CAR T cell therapy. AACR
Arab S, Hadjati J (2019) Adenosine blockage in tumor microenvironment and improvement of cancer immunotherapy. Immune Netw 19:e23
Arab S, Hasannejad F (2021) An overview of current therapeutic strategies for glioblastoma and the role of CD73 as an alternative curative approach. Clin Transl Oncol 24:1–15
Arab S, Alizadeh A, Asgharzade S (2021) Tumor-resident adenosine-producing mesenchymal stem cells as a potential target for cancer treatment. Clin Exp Med 21:205–213
Arabameri A, Asemani D, Hajati J (2018) Mathematical model of cancer immunotherapy by dendritic cells combined with tumor hypoxia treatment. In: 2018 25th National and 3rd International Iranian Conference on Biomedical Engineering (ICBME), IEEE, pp 1–6
Arabameri A, Pourgholaminejad A (2021) Modeling codelivery of CD73 inhibitor and dendritic cell-based vaccines in cancer immunotherapy. Comput Biol Chem 95:107585
Bhat R, Watzl C (2007) Serial killing of tumor cells by human natural killer cells—enhancement by therapeutic antibodies. PLoS ONE 2:e326. https://doi.org/10.1371/journal.pone.0000326
Billeskov R, Beikzadeh B, Berzofsky JA (2019) The effect of antigen dose on T cell-targeting vaccine outcome. Hum Vaccin Immunother 15:407–411
Brady-Nicholls R et al (2020) Prostate-specific antigen dynamics predict individual responses to intermittent androgen deprivation. Nat Commun 11:1750. https://doi.org/10.1038/s41467-020-15424-4
Brentjens RJ et al (2013) CD19-targeted T cells rapidly induce molecular remissions in adults with chemotherapy-refractory acute lymphoblastic leukemia. Sci Transl Med 5:177ra138
Castillo-Montiel E, Chimal-Eguia JC, Tello JI, Piñon-Zaráte G, Herrera-Enríquez M, Castell-Rodríguez A (2015) Enhancing dendritic cell immunotherapy for melanoma using a simple mathematical model. Theor Biol Med Model 12:1–14
Chaudhury A, Zhu X, Chu L, Goliaei A, June CH, Kearns JD, Stein AM (2020) Chimeric antigen receptor T cell therapies: a review of cellular kinetic-pharmacodynamic modeling approaches. J Clin Pharmacol 60:S147–S159
Chen X et al (2016) A combinational therapy of EGFR-CAR NK cells and oncolytic herpes simplex virus 1 for breast cancer brain metastases. Oncotarget 7:27764
Crooks A, Castle C, Batty M (2008) Key challenges in agent-based modelling for geo-spatial simulation. Comput Environ Urban Syst 32:417–430
Das P, Mukherjee S, Das P (2019) An investigation on Michaelis-Menten kinetics based complex dynamics of tumor-immune interaction. Chaos, Solitons Fractals 128:297–305
Das P, Upadhyay RK, Das P, Ghosh D (2020) Exploring dynamical complexity in a time-delayed tumor-immune model. Chaos Interdiscip J Nonlinear Sci 30:123118
de Pillis LG, Gu W, Radunskaya AE (2006) Mixed immunotherapy and chemotherapy of tumors: modeling, applications and biological interpretations. J Theor Biol 238:841–862
de Pillis L et al (2009) Mathematical model creation for cancer chemo-immunotherapy. Comput Math Methods Med 10:571494. https://doi.org/10.1080/17486700802216301
Djema W, Bonnet C, Mazenc F, Clairambault J, Fridman E, Hirsch P, Delhommeau F (2018) Control in dormancy or eradication of cancer stem cells: Mathematical modeling and stability issues. J Theor Biol 449:103–123
Franssen LC, Lorenzi T, Burgess AE, Chaplain MA (2019) A mathematical framework for modelling the metastatic spread of cancer. Bull Math Biol 81:1965–2010
Gaggero S, Witt K, Carlsten M, Mitra S (2020) Cytokines orchestrating the natural killer-myeloid cell crosstalk in the tumor microenvironment: implications for natural killer cell-based cancer immunotherapy. Front Immunol 11:621225. https://doi.org/10.3389/fimmu.2020.621225
Glodde N et al. (2019) Experimental and stochastic models of melanoma T-cell therapy define impact of subclone fitness on selection of antigen loss variants. bioRxiv:860023
Hardiansyah D, Ng CM (2019) Quantitative systems pharmacology model of chimeric antigen receptor T-cell therapy. Clin Transl Sci 12:343–349
Hashmi AA et al (2019) Epidermal growth factor receptor (EGFR) overexpression in triple-negative breast cancer: association with clinicopathologic features and prognostic parameters. Surg Exp Pathol 2:6. https://doi.org/10.1186/s42047-018-0029-0
Hu Z (2020) Tissue factor as a new target for CAR-NK cell immunotherapy of triple-negative breast cancer. Sci Rep 10:1–13
Hu Z, Xu X, Wei H (2021) The adverse impact of tumor microenvironment on NK-cell. Front Immunol 12:2161
Iman RL. Latin hypercube sampling. In: Wiley StatsRef: Statistics Reference Online. doi:https://doi.org/10.1002/9781118445112.stat03803
Jadidi-Niaragh F et al (2017) CD73 specific siRNA loaded chitosan lactate nanoparticles potentiate the antitumor effect of a dendritic cell vaccine in 4T1 breast cancer bearing mice. J Control Release 246:46–59
Kheshtchin N, Hadjati J (2022) Targeting hypoxia and hypoxia-inducible factor-1 in the tumor microenvironment for optimal cancer immunotherapy. J Cell Physiol 237:1285–1298
Kheshtchin N, Arab S, Hadjati J (2019) Blockade of hypoxia: the impact on tumor growth in an experimental tumor model. Immunoregulation 2:35–40
Kimmel GJ, Locke FL, Altrock PM (2021) The roles of T cell competition and stochastic extinction events in chimeric antigen receptor T cell therapy. Proc R Soc B 288:20210229
Kloess S, Kretschmer A, Stahl L, Fricke S, Koehl U (2019) CAR-expressing natural killer cells for cancer retargeting. Transfus Med Hemother 46:4–13
Kondelkova K, Vokurková D, Krejsek J, Borská L, Fiala Z, Ctirad A (2010) Regulatory T cells (TREG) and their roles in immune system with respect to immunopathological disorders. Acta Medica (hradec Kralove) 53:73–77
Kronik N, Kogan Y, Vainstein V, Agur Z (2008) Improving alloreactive CTL immunotherapy for malignant gliomas using a simulation model of their interactive dynamics. Cancer Immunol Immunother 57:425–439
Liu Z, Yang C (2016) A mathematical model of cancer treatment by radiotherapy followed by chemotherapy. Math Comput Simul 124:1–15
Liu E et al (2020a) Use of CAR-transduced natural killer cells in CD19-positive lymphoid tumors. N Engl J Med 382:545–553
Liu Y et al (2020b) Targeting epidermal growth factor-overexpressing triple-negative breast cancer by natural killer cells expressing a specific chimeric antigen receptor. Cell Prolif 53:e12858
Liu C et al (2021) Model-based cellular kinetic analysis of chimeric antigen receptor-T cells in humans. Clin Pharmacol Ther 109:716–727
Liu S, Nguyen K, Park D, Wong N, Wang A, Zhou Y (2022) Harnessing natural killer cells to develop next-generation cellular immunotherapy. Chron Dis Transl Med 8(4):245–255
Macal CM, North MJ (2005) Tutorial on agent-based modeling and simulation. In: Proceedings of the winter simulation conference, 2005. IEEE, p 14 pp.
Macfarlane FR, Lorenzi T, Chaplain MA (2018) Modelling the immune response to cancer: an individual-based approach accounting for the difference in movement between inactive and activated T cells. Bull Math Biol 80:1539–1562
Mahlbacher G, Curtis LT, Lowengrub J, Frieboes HB (2018) Mathematical modeling of tumor-associated macrophage interactions with the cancer microenvironment. J Immunother Cancer 6:1–17
Marino S, Hogue IB, Ray CJ, Kirschner DE (2008) A methodology for performing global uncertainty and sensitivity analysis in systems biology. J Theor Biol 254:178–196
Marofi F et al (2021) CAR-NK cell: a new paradigm in tumor immunotherapy. Front Oncol 11:2078
McMichael EL et al (2017) IL-21 enhances natural killer cell response to cetuximab-coated pancreatic tumor cells. Clin Cancer Res 23:489–502
Mirsanei Z et al (2020) Optimized dose of dendritic cell-based vaccination in experimental model of tumor using artificial neural network. Iran J Allergy and Immunol 19:172–182
Mostolizadeh R, Afsharnezhad Z, Marciniak-Czochra A (2018) Mathematical model of chimeric anti-gene receptor (CAR) T cell therapy with presence of cytokine. Numer Algebra Control Optim 8:63
Nukala U, Rodriguez Messan M, Yogurtcu ON, Wang X, Yang H (2021) A systematic review of the efforts and hindrances of modeling and simulation of CAR T-cell therapy. AAPS J 23:1–20
Portillo AL et al (2021) Expanded human NK cells armed with CAR uncouple potent anti-tumor activity from off-tumor toxicity against solid tumors. Iscience 24:102619
Qian G, Mahdi A (2020) Sensitivity analysis methods in the biomedical sciences. Math Biosci 323:108306
Rezvani K, Rouce R, Liu E, Shpall E (2017) Engineering natural killer cells for cancer immunotherapy. Mol Ther 25:1769–1781
Rodriguez-Brenes IA, Komarova NL, Wodarz D (2013) Tumor growth dynamics: insights into evolutionary processes. Trends Ecol Evol 28:597–604
Romee R, Leong JW, Fehniger TA (2014) Utilizing cytokines to function-enable human NK cells for the immunotherapy of cancer. Scientifica. Vol. 2014
Sahoo P et al (2020) Mathematical deconvolution of CAR T-cell proliferation and exhaustion from real-time killing assay data. J R Soc Interface 17:20190734
Shemesh A, Pickering H, Roybal KT, Lanier LL (2022) Differential IL-12 signaling induces human natural killer cell activating receptor-mediated ligand-specific expansion. J Exp Med. https://doi.org/10.1084/jem.20212434
Shevtsov M et al (2019) Ex vivo Hsp70-activated NK cells in combination with PD-1 inhibition significantly increase overall survival in preclinical models of glioblastoma and lung cancer. Front Immunol 10:454
Singh AP, Chen W, Zheng X, Mody H, Carpenter TJ, Zong A, Heald DL (2021) Bench-to-bedside translation of chimeric antigen receptor (CAR) T cells using a multiscale systems pharmacokinetic-pharmacodynamic model: a case study with anti-BCMA. CAR-T CPT Pharmacomet Syst Pharmacol 10:362–376
Singh AP et al. Development of a quantitative relationship between CAR-affinity, antigen abundance, tumor cell depletion and CAR-T cell expansion using a multiscale systems PK-PD model. In: MAbs, 2020. vol 1. Taylor & Francis, p 1688616
Storey KM, Jackson TL (2021) An agent-based model of combination oncolytic viral therapy and anti-PD-1 immunotherapy reveals the importance of spatial location when treating glioblastoma. Cancers 13:5314
Suzuki C et al (2012) The initial change in tumor size predicts response and survival in patients with metastatic colorectal cancer treated with combination chemotherapy. Ann Oncol 23:948–954
Tanaka A, Sakaguchi S (2019) Targeting Treg cells in cancer immunotherapy. Eur J Immunol 49:1140–1146
Wang R, Bao W, Pal M, Liu Y, Yazdanbakhsh K, Zhong H (2022) Intermediate monocytes induced by IFN-γ inhibit cancer metastasis by promoting NK cell activation through FOXO1 and interleukin-27. J Immunother Cancer 10:e003539. https://doi.org/10.1136/jitc-2021-003539
Wherry EJ, Kurachi M (2015) Molecular and cellular insights into T cell exhaustion. Nat Rev Immunol 15:486–499
Wilson S, Levy D (2012) A mathematical model of the enhancement of tumor vaccine efficacy by immunotherapy. Bull Math Biol 74:1485–1500
Wu G, Wu J, Wang B, Zhu X, Shi X, Ding Y (2018) Importance of tumor size at diagnosis as a prognostic factor for hepatocellular carcinoma survival: a population-based study. Cancer Manag Res 10:4401
Yada E, Wada S, Yoshida S, Sasada T (2017) Use of patient-derived xenograft mouse models in cancer research and treatment. Future Sci 4:FS2O71
Yang HM (2012) Mathematical modeling of solid cancer growth with angiogenesis. Theor Biol Med Model 9:1–39
Yokota J (2000) Tumor progression and metastasis. Carcinogenesis 21:497–503. https://doi.org/10.1093/carcin/21.3.497
Zand B et al (2022) Identification of the optimal pattern of the injection and dosage of DC immunotherapy using the mathematical models based on ordinary differential equations. Iran J Immunol 19:1–17
Zhang C, Hu Y, Xiao W, Tian Z (2021) Chimeric antigen receptor-and natural killer cell receptor-engineered innate killer cells in cancer immunotherapy. Cell Mol Immunol 18:2083–2100
Zhao J et al (2020) Single-cell RNA sequencing reveals the heterogeneity of liver-resident immune cells in human. Cell Discov 6:22. https://doi.org/10.1038/s41421-020-0157-z
Zimmer J, Andrès E, Hentges F (2008) NK cells and Treg cells: a fascinating dance cheek to cheek. Eur J Immunol 38:2942–2945. https://doi.org/10.1002/eji.200838813
Funding
This research did not receive any specific grant from funding agencies in the public, commercial, or not-for-profit sectors.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflicts of interest
The authors declare that there is no conflict of interest.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Arabameri, A., Arab, S. Understanding the Interplay of CAR-NK Cells and Triple-Negative Breast Cancer: Insights from Computational Modeling. Bull Math Biol 86, 20 (2024). https://doi.org/10.1007/s11538-023-01247-z
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s11538-023-01247-z