Abstract
Cancer stem cells (CSCs) drive tumor progression, metastases, treatment resistance, and recurrence. Understanding CSC kinetics and interaction with their nonstem counterparts (called tumor cells, TCs) is still sparse, and theoretical models may help elucidate their role in cancer progression. Here, we develop a mathematical model of a heterogeneous population of CSCs and TCs to investigate the proposed “tumor growth paradox”—accelerated tumor growth with increased cell death as, for example, can result from the immune response or from cytotoxic treatments. We show that if TCs compete with CSCs for space and resources they can prevent CSC division and drive tumors into dormancy. Conversely, if this competition is reduced by death of TCs, the result is a liberation of CSCs and their renewed proliferation, which ultimately results in larger tumor growth. Here, we present an analytical proof for this tumor growth paradox. We show how numerical results from the model also further our understanding of how the fraction of cancer stem cells in a solid tumor evolves. Using the immune system as an example, we show that induction of cell death can lead to selection of cancer stem cells from a minor subpopulation to become the dominant and asymptotically the entire cell type in tumors.









Similar content being viewed by others
References
Al-Hajj, M., Wicha, M. S., Benito-Hernandez, A., Morrison, S. J., & Clarke, M. F. (2003). Prospective identification of tumorigenic breast cancer cells. Proc. Natl. Acad. Sci. USA, 100(7), 3983–3988.
Alarcon, T., Owen, M. R., Byrne, H. M., & Maini, P. K. (2006). Multiscale modelling of tumour growth and therapy: the influence of vessel normalisation on chemotherapy. Comput. Math. Methods Med., 7(2–3), 85–119.
Almog, N., Ma, L., Raychowdhury, R., Schwager, C., Erber, R., Short, S., Hlatky, L., Vajkoczy, P., Huber, P. E., Folkman, J., & Abdollahi (2009). A transcriptional switch of dormant tumors to fast-growing angiogenic phenotype. Cancer Res., 69(3), 836–844.
Anderson, A. R. A., Weaver, A. M., Cummings, P. T., & Quaranta, V. (2006). Tumor morphology and phenotypic evolution driven by selective pressure from the microenvironment. Cell, 127, 905–915.
Barcellos-Hoff, M. H. (2001). It takes a tissue to make a tumor: epigenetics, cancer and the microenvironment. J. Mammary Gland Biol. Neoplasia, 6(2), 213–221.
Bellomo, N., & Delitala, M. (2008). From the mathematical kinetic, and stochastic game theory to modelling mutations, onset, progression and immune competition of cancer cells. Phys. Life Rev., 5, 183–206.
Bonnet, D., & Dick, J. E. (1997). Human acute myeloid leukemia is organized as a hierarchy that originates from a primitive hematopoietic cell. Nat. Med., 3, 730–737.
Cammareri, P., Lombardo, Y., Francipane, M. G., et al. (2008). Isolation and culture of colon cancer stem cells. Methods Cell Biol., 86, 311–324.
Conde-Ramis, I., Drasdo, D., Anderson, A. R. A., & Chaplain, M. A. J. (2008). Modelling the influence of the e-cadherin—beta-catenin pathway in cancer cell invasion: A multi-scale approach. Biophys. J., 95(1), 155–165.
de Pillis, L., & Radunskaya, A. (2001). A mathematical tumor model with immune resistance and drug therapy: an optimal control approach. J. Theor. Med., 3, 79–100.
DeLisi, C., & Rescigno, A. (1977). Immune surveillance and neoplasia—I: a minimal mathematical model. Bull. Math. Biol., 39(2), 201–221.
Dick, J. E. (2003). Breast cancer stem cells revealed. Proc. Natl. Acad. Sci. USA, 100(7), 3547–3549.
Dingli, D., & Michor, F. (2006). Successful therapy must eradicate cancer stem cells. Stem Cells, 24(12), 2603–2610.
D’Onofrio, A. (2005). A general framework for modeling tumor-immune system competition and immunotherapy: Mathematical analysis and biomedical inferences. Physica D, 208, 220–235.
Dunn, G. P., Bruce, A. T., Ikeda, H., Old, L. J., & Schreiber, R. D. (2002). Cancer immunoediting: from immunosurveillance to tumor escape. Nat. Immunol., 3(11), 991–998.
Dunn, G. P., Old, L. J., & Schreiber, R. D. (2004). The three E’s of cancer immunoediting. Annu. Rev. Immunol., 22, 329–360.
Enderling, H., Alexander, N., Clark, E., et al. (2008). Dependence of invadopodia function on collagen fiber spacing and crosslinking: computational modeling and experimental evidence. Biophys. J., 95(5), 2203–2218.
Enderling, H., Anderson, A. R. A., Chaplain, M. A. J., Beheshti, A., Hlatky, L., & Hahnfeldt, P. (2009). Paradoxical dependencies of tumor dormancy and progression on basic cell kinetics. Cancer Res., 69(22), 8814–8821.
Enderling, H., Hlatky, L., & Hahnfeldt, P. (2009b). Migration rules: tumours are conglomerates of self-metastases. Br. J. Cancer, 100(12), 1917–1925.
Fioriti, D., Mischitelli, M., Di Monaco, F., et al. (2008). Cancer stem cells in prostate adenocarcinoma: a target for new anticancer strategies. J. Cell. Physiol., 216(3), 571–575.
Folkman, J. (1971). Tumor angiogenesis: therapeutic implications. N. Engl. J. Med., 285(21), 1182–1186.
Folkman, J., & Hanahan, D. (1991). Switch to the angiogenic phenotype during tumorigenesis. In Int. symp. Princess Takamatsu Cancer Res. Fund (Vol. 22, pp. 339–347).
Ganguli, R., & Puri, I. K. (2006). Mathematical model for the cancer stem cell hypothesis. Cell Prolif., 39, 3–14.
Gatenby, R. A., & Gillies, R. J. (2008). A microenvironmental model of carcinogenesis. Nat. Rev. Cancer, 8(1), 56–61.
Gevertz, J. L., & Torquato, S. (2006). Modeling the effects of vasculature evolution on early brain tumor growth. J. Theor. Biol., 243(4), 517–531.
Greese, B. (2006). Development, analysis and application of a nonlinear integro-differential equation in the context of cancer growth. Diplomarbeit (MSc thesis), University of Greifswald, Germany, and University of Alberta.
Hahnfeldt, P., Panigrahy, D., Folkman, J., & Hlatky, L. (1999). Tumor development under angiogenic signaling: a dynamical theory of tumor growth, treatment response, and postvascular dormancy. Cancer Res., 59(19), 4770–4775.
Hanahan, D., & Weinberg, R. (2000). The hallmarks of cancer. Cell, 100(1), 57–70.
Hanahan, D., & Weinberg, R. A. (2011). Hallmarks of cancer: The next generation. Cell, 144, 646–674.
Hek, G. (2010). Geometric singular perturbation theory in biological practice. J. Math. Biol., 60(3), 347–386.
Johnston, M. D., Maini, P. K., Chapman, S. J., Edwards, C. M., & Bodmer, W. F. (2010). On the proportion of cancer stem cells in a tumour. J. Theor. Biol., 266, 708–711.
Jones, C. K. R. T. (1994). Geometric singular perturbation theory. In J. Russell (Ed.), Dynamical systems, Montecatini Terme, Italy, 1994. Berlin: Springer. 2nd session of the Centro Internazionale Matematico Estivo (CIME).
Kim, M.-Y., Oskarsson, T., Acharrya, S., et al. (2009). Tumor self-seeding by circulating cancer cells. Cell, 139(7), 1315–1326.
Kirschner, D., & Panetta, J. C. (1998). Modeling immunotherapy of the tumor-immune interaction. J. Math. Biol., 37(3), 235–252.
Kuznetsov, V. (1987). Mathematical modeling of the development of dormant tumors and immune stimulation of their growth. Cybern. Syst. Anal., 23(4), 556–564.
Lapidot, T., Sirard, C., Murdoch, B., et al. (1994). A cell initiating human acute myeloid leukaemia after transplantation into scid mice. Nature, 367(6464), 645–648.
Liu, W., Hillen, T., & Freedman, H. I. (2007). A mathematical model for M-phase specific chemotherapy including the G0-phase and immunoresponse. Math. Biosci. Eng., 4(2), 239–259.
Maitland, N. J., & Colling, T. (2008). Prostate cancer stem cells: a new target for therapy. J. Clin. Oncol., 26(17), 2862–2870.
Mallet, D. G., & De Pillis, L. G. (2006). A cellular automata model of tumor-immune system interactions. J. Theor. Biol., 239(3), 334–350.
Marciniak-Czochra, A., Stiehl, T., Ho, A. D., Jäger, W., & Wagner, W. (2009). Modelling of asymmetric cell division in hematopoietic stem cells—regulation of self-renewal is essential for efficient repopulation. Stem Cells Dev., 18(3), 377–385.
Matzavinos, A., & Chaplain, M. A. J. (2004). Travelling-wave analysis of a model of the immune response to cancer. C. R. Biol., 327, 995–1008.
Morrison, S. J., & Kimble, J. (2006). Asymmetric and symmetric stem-cell divisions in development and cancer. Nature, 441(7097), 1068–1074.
Morton, C. I., Hlatky, L., Hahnfeldt, P., & Enderling, H. (2011). Non-stem cancer cell kinetics modulate solid tumor progression. Theor. Biol. Med. Model., 8(1), 48.
Naumov, G. N., Bender, E., Zurakowski, D., et al. (2006). A model of human tumor dormancy: an angiogenic switch from the nonangiogenic phenotype. J. Natl. Cancer Inst., 98(5), 316–325.
Norton, L. (2005). Conceptual and practical implications of breast tissue geometry: Toward a more effective, less toxic therapy. The Oncologist, 10(6), 370–381.
Prehn, R. T. (1972). The immune reaction as a stimulator of tumor growth. Science, 176(4031), 170–171.
Prehn, R. T. (1991). The inhibition of tumor growth by tumor mass. Cancer Res., 51(1), 2–4.
Quaranta, V., Rejniak, K. A., Gerlee, P., & Anderson, A. R. A. (2008). Invasion emerges from cancer cell adaptation to competitive microenvironments: quantitative predictions from multiscale mathematical models. Semin. Cancer Biol., 18(5), 338–348.
Quaranta, V., Weaver, A. M., Cummings, P. T., & Anderson, A. R. A. (2005). Mathematical modeling of cancer: the future of prognosis and treatment. Clin. Chim. Acta, 357(2), 173–179.
Quintana, E., Shackleton, M., Sabel, M. S., Fullen, D. R., Johnson, T. M., & Morrison, S. J. (2008). Efficient tumour formation by single human melanoma cells. Nature, 456(7222), 593–598.
Reya, R., Morrison, S. J., Clarke, M. F., & Weissman, I. L. (2001). Stem cells, cancer, and cancer stem cells. Nature, 414(6859), 105–111.
Ribba, B., Alarcon, T., Marron, K., Maini, P. K., & Agur, Z. (2004). The use of hybrid cellular automaton models for improving cancer therapy. Lect. Notes Comput. Sci., 3305, 444–453.
Schatton, T., & Frank, M. H. (2009). Antitumor immunity and cancer stem cells. Ann. N.Y. Acad. Sci., 1176, 154–169.
Sherratt, J. A., & Nowak, M. A. (1992). Oncogenes, anti-oncogenes and the immune response to cancer: a mathematical model. Proc. Biol. Sci., 248(1323), 261–271.
Singh, S. K., Clarke, I. D., Terasaki, M., et al. (2004). Identification of human brain tumour initiating cells. Nature, 432(7015), 396–401.
Singh, S. K., Hawkins, C., Clarke, I. D., et al. (2003). Identification of a cancer stem cell in human brain tumors. Cancer Res., 63(18), 5821–5828.
Smallbone, K., Gatenby, R. A., Gillies, R. J., Maini, P. K., & Gavaghan, D. J. (2007). Metabolic changes during carcinogenesis: potential impact on invasiveness. J. Theor. Biol., 244(4), 703–713.
Sole, R. V., Rodriguez-Caso, C., Diesboeck, T. S., & Saldance, J. (2008). Cancer stem cells as the engine of unstable tumor progression. J. Theor. Biol., 253.
Teng, W. L., Swann, J. B., Koebel, C. M., Schreiber, R. D., & Smyth, M. J. (2008). Immune-mediated dormancy: an equilibrium with cancer. J. Leukoc. Biol., 84(4), 988–993.
Todaro, M., Perez Alea, M., Di Stefano, A. B., et al. (2007). Colon cancer stem cells dictate tumor growth and resist cell death by production of interleukin-4. Cell Stem Cell, 1(4), 389–402.
Visvader, J., & Lindeman, G. J. (2008). Cancer stem cells in solid tumours: accumulating evidence and unresolved questions. Nat. Rev. Cancer, 8(10), 755–768.
Wang, Z. A., & Hillen, T. (2007). Classical solutions and pattern formation for a volume filling chemotaxis model. Chaos, 17, 037108. 13 pages.
Wise, S. M., Lowengrub, J. A., Frieboes, H. B., & Cristini, V. (2008). Three-dimensional multispecies nonlinear tumor growth—I. J. Theor. Biol., 253, 524–543.
Youssefpour, H., Li, X., Lander, A. D., & Lowengrub, J. S. (2012). Multispecies model of cell lineages and feedback control in solid tumors. J. Theor. Biol., 304, 39–59.
Acknowledgements
The authors wish to thank Gerda de Vries and Jeff Bachman for fruitful discussions and remarks. The work of TH was supported by the Canadian NSERC. The work of HE was supported by the American Association for Cancer Research award number 08-40-02-ENDE (to HE) and the work of HE and PH was supported by the Office of Science (BER), US Department of Energy, under Award Number DE-SC0001434 (to PH).
Author information
Authors and Affiliations
Corresponding author
Appendix: Equivalence of Basic Stem Cell Models
Appendix: Equivalence of Basic Stem Cell Models
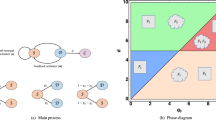
Here, we show that the three models for cancer stem cells that are illustrated in Fig. 2 are equivalent in the situation where the stem cell population is not declining. Let U(t) and V(t) denote the CSC and TC density at time t, and k the rate of CSC division. For the purpose of demonstrating this equivalence, we ignore TC divisions. We first describe a hypothetical “complete model” (i) that has all three features, then demonstrate that dropping feature (ii) maintains model generality, while dropping feature (iii) also maintains generality provided parameters are chosen in the complete model such that the CSC compartment never decreases in time.
Complete Model
We introduce the complete model that includes all three division fates described above. Let α 1 denote the fraction of symmetric division, α 2 the fraction of asymmetric division, and α 3 the fraction of symmetric commitment events, with α 1+α 2+α 3=1. A schematic is shown on the left in Fig. 2.
The change in cell populations due to CSC division events can then be described by:

Invoking the identity α 1=1−α 2−α 3, we obtain the system
where α 2+2α 3≠(0,1) and α 2+2α 3<1 (or equivalently, α 1>α 3). The last condition arises from the assumption that the number of CSCs does not decrease in time.
No Symmetric Commitment Model
This model assumes that CSC is a robust state that cannot be lost during mitosis (Enderling et al. 2009). Therefore, the dividing CSC always remains CSC, and the second daughter cell is either a CSC or a TC (Fig. 2). This model is the Complete Model with the additional condition of no chance of commitment, i.e., α 3=0. From a simple inspection of Equation System (27) with α 3=0, though, we see this model remains just as general as the Complete Model, since the leading coefficients on the right sides of the equations for U and V can range from 0 to 1 as before.
No Asymmetric Division Model
The model most often used in the literature ignores asymmetric CSC division (Ganguli and Puri 2006; Marciniak-Czochra et al. 2009; Wise et al. 2008). A mitotic CSC event either yields two CSC or two TC (Fig. 2). This model is the Complete Model with the additional condition of no chance of asymmetric division, i.e., α 2=0. From a simple inspection of Equation System (27) with α 2=0, though, we see this model remains just as general as the Complete Model, since the leading coefficients on the right sides of the equations for U and V can range from 0 to 1 as before.
In summary, we have shown that the “No Symmetric Commitment” and “No Asymmetric Division” models are individually equivalent to the “Complete Model,” and so to each other. We therefore discuss a mathematical model that essentially exploits the “No Symmetric Commitment” model above, with the appreciation that it will not only provide analytic confirmation of the tumor growth paradox revealed by our agent-based studies (Enderling et al. 2009), but will simultaneously confirm the applicability of various sets of cell division rules we could alternatively have employed to build the model.
Rights and permissions
About this article
Cite this article
Hillen, T., Enderling, H. & Hahnfeldt, P. The Tumor Growth Paradox and Immune System-Mediated Selection for Cancer Stem Cells. Bull Math Biol 75, 161–184 (2013). https://doi.org/10.1007/s11538-012-9798-x
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11538-012-9798-x