Abstract
Background
Multimodal treatment of newly diagnosed high-risk neuroblastoma (HRNB) includes induction chemotherapy, consolidation with myeloablative therapy (MAT) and autologous stem cell transplantation (ASCT), followed by anti-disialoganglioside 2 (GD2) immunotherapy, as recommended by the Children’s Oncology Group (COG) and the Society of Paediatric Oncology European Neuroblastoma (SIOPEN). Some centres proposed an alternative approach with induction chemotherapy followed by anti-GD2 immunotherapy, without MAT+ASCT.
Objective
The aim of this systematic literature review was to compare survival outcomes in patients with HRNB treated with or without MAT+ASCT and with or without subsequent anti-GD2 immunotherapy.
Patients and Methods
The review followed the Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) guidelines. MEDLINE via PubMed and EMBASE databases were systematically searched for randomised controlled trials (RCT) and observational comparative studies in patients with HRNB using search terms for ‘neuroblastoma’ and (‘myeloablative therapy’ OR ‘stem cell transplantation’). Reporting of at least one survival outcome [event-free survival (EFS), progression-free survival, relapse-free survival and/or overall survival (OS)] was required for inclusion. Outcomes from RCTs were synthesized in meta-analysis, while meta-analysis of non-RCTs was not planned owing to expected heterogeneity.
Results
Literature searches produced 2587 results with 41 publications reporting 34 comparative studies included in the review. Of these, 7 publications reported 4 RCTs, and 34 publications reported 30 non-RCT studies. Studies differed with respect to included populations, induction regimen, response to induction, additional treatments and transplantation procedures. Subsequent treatments of relapse were rarely reported and could not be compared. In the meta-analysis, EFS was in favour of MAT+ASCT over conventional chemotherapy or no further treatment [hazard ratio (HR) = 0.78, 95% confidence interval (CI) 0.67−0.91, p = 0.001] with a trend favouring MAT+ASCT for OS (HR = 0.86, 95% CI 0.73−1.00, p = 0.05). Tandem MAT+ASCT was found to improve EFS compared with the single procedure, with improvement in both EFS and OS in patients treated with anti-GD2 therapy. Non-RCT comparative studies were broadly consistent with evidence from the RCTs; however, not all reported survival benefits of MAT+ASCT (single or tandem). Limited comparative evidence on treatment without MAT+ASCT in patients treated with anti-GD2 immunotherapy suggests an increased risk of relapse. In relapsed patients, MAT+ASCT appears to improve OS, but evidence remains scarce.
Conclusions
Survival benefits in patients treated with MAT+ASCT confirm that the procedure should remain an integral part of multimodal therapy. In patients treated with anti-GD2 immunotherapy, limited evidence suggests that omitting MAT+ASCT is associated with an increased risk of relapse, and therefore, a change in clinical practice can currently not be recommended. Evidence suggests the use of tandem MAT+ASCT compared with the single procedure, with greater benefits observed in patients treated with anti-GD2 immunotherapy. Limited evidence also suggests improved survival following MAT+ASCT in relapsed patients, which needs to be viewed in light of emerging chemoimmunotherapy in this setting.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
In patients with high-risk neuroblastoma (HRNB) not treated with anti-disialoganglioside 2 (GD2) immunotherapy during maintenance, myeloablative therapy (MAT) + autologous stem cell transplantation (ASCT) improves event-free survival (EFS) with a favourable trend for overall survival (OS) compared with conventional chemotherapy or no further treatment. |
In patients with HRNB treated with anti-GD2 immunotherapy, tandem MAT+ASCT appears to improve both EFS and OS. |
Limited comparative evidence on treatment without MAT+ASCT but with maintenance anti-GD2 immunotherapy suggests an increased risk of relapse, and currently does not warrant a change in clinical practice. |
1 Introduction
Neuroblastoma, a malignancy of the peripheral sympathetic nervous system, is the most common extracranial paediatric solid tumour [1], with the majority of children diagnosed before the age of 5 years [2]. It accounts for 8–10% of paediatric cancer cases and 15% of cancer deaths in children [3, 4]. Approximately half of the patients diagnosed with neuroblastoma have a clinically aggressive form—high-risk neuroblastoma (HRNB) [2, 5].
Current treatment regimens for patients with newly diagnosed HRNB comprise (1) induction chemotherapy followed by surgical resection of the primary tumour to reduce the tumour burden as much as possible and (2) consolidation therapy with myeloablative therapy (MAT) and autologous stem cell transplantation (ASCT), followed by radiotherapy and maintenance therapy. Maintenance therapy consists of immunotherapy with an anti-disialoganglioside 2 (GD2) monoclonal antibody, such as dinutuximab or dinutuximab beta, as recommended by the Children’s Oncology Group (COG) and the Society of Paediatric Oncology European Neuroblastoma (SIOPEN) [6], plus isotretinoin (13-cis-retinoic acid) [2, 5]. This intense multimodal therapy takes approximately 18 months [5], and has increased survival of patients with HRNB from under 15% to over 50% with the introduction of ASCT and over 60% with immunotherapy, thus, markedly improving their chance of cure [6,7,8,9].
MAT is intended to consolidate the response to induction therapy and to eradicate minimal residual disease to reduce the risk of relapse [10]. Following MAT, ASCT enables haematopoietic recovery [11], and hence recovery of the immune system compromised by MAT and required for anti-cancer activity, particularly when followed by anti-GD2 immunotherapy. Subsequent to publication of promising data from early single-arm studies or retrospective analyses indicating the benefit of consolidation therapy with MAT+ASCT [11, 12], the benefit of MAT+ASCT was confirmed in a randomised controlled trial (RCT), the Children’s Cancer Group (CCG)-3891 study, in which 3-years event-free survival (EFS) significantly improved versus continued intensive chemotherapy [13]. As a result of these findings, MAT+ASCT has been an integral part of the multimodal first-line therapy for HRNB for over 2 decades. Long-term follow-up of the CCG-3891 study population confirmed the earlier results, with a significantly higher 5-years EFS rate [10].
Maintenance with anti-GD2 immunotherapy has been part of the multimodal treatment of HRNB in Europe and the USA for over a decade. The efficacy of this approach was demonstrated in large co-operative trials with COG (ANBL0032) [14] and SIOPEN (HR-NBL1/SIOPEN) [15], in which all patients had undergone MAT+ASCT prior to maintenance therapy. In the HR-NBL1/SIOPEN trial, the effects of two different MAT regimens were investigated, demonstrating improved survival on busulfan/melphalan (BuMel) versus carboplatin/etoposide/melphalan (CEM) [16]. The COG also undertook an RCT to determine whether intensifying consolidation treatment with tandem ASCTs (sequential courses of MAT+ ASCTs) would improve survival compared with a single ASCT in patients with HRNB (ANBL0532) [17]. Establishing MAT+ASCT as the standard of care made it unethical to randomise to alternative treatments without MAT+ASCT, so the existing randomised evidence included only patients who had undergone this consolidation procedure. While the use of MAT+ASCT offers a survival benefit in patients with HRNB [18], MAT is associated with significant adverse events, some of which have a long-term impact, such as secondary malignant cancers [19,20,21], deafness and infertility [22]. The side effects in neuroblastoma survivors also occur in children treated without MAT, especially deafness after cisplatin or ovarian failure after high-dose cyclophosphamide [23,24,25]. MAT, especially BuMel, may be associated with a higher risk of veno-occlusive disease early during treatment [26]. Whereas the most severe Grade 3 and 4 side effect associated with BuMel is veno-occlusive disease, the most frequently reported Grade 3 and 4 side effects with CEM are poor general condition, infections and stomatitis [16]. Furthermore, MAT can only be administered in patients who can undergo subsequent ASCT to restore haematopoiesis and immune function, and while access to ASCT has been increasing worldwide, it remains scarce in some lower-income countries, often owing to limited funds and infrastructure [27].
In contrast to centres which have adopted MAT+ASCT as standard of care, at the Memorial Sloan Kettering Cancer Centre (MSKCC), since 2003, patients with HRNB have received induction chemotherapy followed by anti-GD2 immunotherapy plus granulocyte-macrophage colony-stimulating factor (GM-CSF), local radiotherapy, and isotretinoin, but without MAT+ASCT [28, 29]. This approach was based on retrospective early data suggesting lack of survival advantage following treatment with MAT+ASCT versus maintenance chemotherapy [30, 31]. It was hypothesised that, in the era of anti-GD2 immunotherapy, MAT+ASCT may no longer improve survival and that it should be abandoned [28]. In a non-randomised comparison, there was no statistically significant difference in 5-year EFS or 5-year overall survival (OS) between patients who had previously undergone MAT+ASCT and those who received conventional chemotherapy followed by anti-GD2 therapy at the MSKCC (2003–2013) [28]. However, OS was likely improved by the use of effective therapies in later lines of treatment, such as chemoimmunotherapy [32, 33]. Indeed, the continued use of MAT+ASCT for the treatment of patients with HRNB has been challenged and debated on the basis of efficacy and safety in two recent articles [34, 35], during a dedicated workshop at the International Society for Paediatric Oncology (SIOP) congress 2021 and by patient advocacy groups [36]. Evidence on efficacy and safety of MAT+ASCT in HRNB was reviewed in 2015 [18], and new evidence has emerged since. Results from the trial of tandem MAT+ASCT were published [17], and haploidentical stem cell transplantation (SCT) has been successfully used in patients with relapsed HRNB [37].
The objective of this systematic literature review was to compare survival outcomes in patients with HRNB treated with MAT+ASCT with those in patients who did not receive MAT+ASCT with or without subsequent anti-GD2 immunotherapy. Specific comparisons considered in this review are shown in Supplemental Table 1, and further rationale for the comparisons is provided in Supplemental Table 2.
2 Methods
This systematic review was conducted in accordance with the Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) guidelines.
2.1 Search Strategy
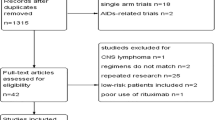
MEDLINE via PubMed and EMBASE electronic databases were systematically searched on 12 April 2023, with 2523 publications retrieved (Fig. 1). The search strategy was informed by the most recent Cochrane review undertaken to compare outcomes of MAT+ASCT versus conventional therapy in children with HRNB [18], which included RCTs only. Our strategy was modified to also include non-randomised comparative studies.
No restrictions regarding the age of the patients or the date of publication were applied, but only publications in English were included. The publication/article types were restricted to clinical studies/trials including Phase II–IV studies, randomised controlled, controlled, pragmatic, comparative or multicentre studies, observational studies, meta-analyses, systematic reviews, guidelines, practice guidelines and published errata. Congress/conference presentations were not included.
The search terms were ‘neuroblastoma’ and (‘myeloablative therapy’ OR ‘stem cell transplantation’). The full search strategies for MEDLINE and EMBASE are provided in Supplemental Table 3, along with the filters applied. The search results were imported into the Rayyan portal for the removal of duplicates and to facilitate screening.
2.2 Data Extraction
Three authors independently screened abstracts and selected publications for inclusion. In cases of discrepancy, the decision was discussed to reach a consensus. Data were extracted from included publications by UZ and JW for analysis. An independent quality assurance check versus the source materials was conducted by Centrum HTA.
2.3 Inclusion Criteria
We evaluated comparative studies of patients with HRNB, regardless of design, where MAT+ASCT was compared with treatment that did not include MAT+ASCT. We considered various MAT and transplantation techniques but excluded studies with metaiodobenzylguanidine (MIBG) only. We also investigated studies comparing allogeneic (including haploidentical) SCT with non-SCT treatments, including historical controls and retrospective analyses. Comparison of different MAT+ASCT regimens or different maintenance regimens was beyond the scope of this review. To be included, at least one comparative survival outcome [EFS, progression-free survival (PFS), relapse-free survival (RFS) and/or OS] should have been reported in the study.
2.4 Outcome Measures
The following survival outcome measures were extracted from publications: EFS (defined as the time to recurrence or progression of disease, death from any cause or secondary neoplasm), PFS (defined as time to death from any cause or relapse/recurrence or progression), RFS (defined as time to death or relapse/recurrence) and OS (time to death from any cause). Survival rates at reported time points and mean and/or median time to event, as well as hazard ratios (HR) along with p-values and confidence intervals (CI), were extracted. If specific survival rates were missing, we used 3- and 5-year data from Kaplan–Meier curves. Subgroup analyses included stratification by anti-GD2 immunotherapy, MYCN status and induction response. Treatments in the first-line setting and in relapsed patients were considered separately.
2.5 Evidence Synthesis and Analyses
Evidence for all studies was reported following the Population, Intervention, Comparison, Outcomes and Study (PICOS) framework along with risk of bias assessment according to Cochrane Handbook for Systematic Reviews of Interventions, using Newcastle–Ottawa Scale for non-RCTs. Outcomes from the RCTs were synthesised in meta-analysis, while meta-analysis for non-RCTs was not planned owing to expected heterogeneity.
The clinical trial presented in Park et al. [17] was excluded from the meta-analysis because of inadequate comparator (transplantation in both arms); however, data on single versus tandem MAT+ASCT were analysed separately. For the assessment of EFS and OS, the generic inverse variance function of RevMan version 5.4.1 was used to combine logs of HR and estimate corresponding 95% CI. HR data (with 95% CI) for studies by Matthay et al. [13] and Matthay et al. [10] and Pritchard et al. [38] were extracted from Yalcin et al. [18]; Yalcin et al. [18] used Parmar’s 1998 method if HR were not reported in the studies]. HR were calculated using the complete follow-up period of the trials.
3 Results
3.1 Identified Publications
PubMed and EMBASE searches produced 629 and 1958 results, respectively (Fig. 1). After removal of 64 duplicate publications, 2523 abstracts were screened, which resulted in 69 publications being identified for full text review. Overall, 41 publications (Supplemental Table 4) reporting 34 comparative studies were included in the systematic review. Of these, 7 publications reported 4 RCTs, and 34 publications reported 30 non-RCT studies.
3.1.1 Included Studies
Four RCTs were identified for the review (Table 1), all of which were multicentre studies. A total of 30 non-RCT studies were identified (Supplemental Table 5); 3 were prospective and 27 retrospective, and 17 studies were multicentre and 13 single centre. There were 22 studies (3 RCTs; 19 non-RCTs) of single MAT+ASCT without subsequent anti-GD2 immunotherapy, 4 non-RCTs studies of MAT+ASCT with subsequent anti-GD2 immunotherapy, 7 studies of tandem versus single MAT+ASCT (1 RCT; 6 non-RCTs), 1 non-RCT study of relapsed populations and no comparative studies reporting survival following allogeneic SCT identified for the review.
Studies differed in terms of patient populations, induction regimens, types of MAT and various treatment procedures. Subsequent relapse treatments were infrequently reported. RCTs generally had low risk of bias, except for blinding, while most non-RCTs were of high quality. Risk of bias assessment is shown in Supplemental Appendix 1.
3.2 MAT+ASCT without Subsequent Anti-GD2 Therapy
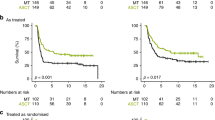
Overall, RCTs provide evidence of the survival benefit of MAT+ASCT versus treatment without MAT+ASCT (Table 1). The meta-analysis of three of the four identified RCTs [10, 13, 38,39,40], which included updated results from the GPOH NB97 study [40], confirmed previously reported results [18]. There was a significant difference in EFS in favour of MAT+ASCT over conventional chemotherapy or no further treatment (HR = 0.78, 95% CI 0.67–0.91, p = 0.001; Fig. 2). There was a non-significant trend in favour of MAT+ASCT over conventional chemotherapy or no further treatment for OS (HR = 0.86, 95% CI 0.73–0.100, p = 0.05; Fig. 3).
A subgroup meta-analysis of EFS, including additional follow-up data and secondary malignant disease as an event (Matthay et al. [13], Berthold et al. [39] and Berthold et al. [40]), showed a significant difference in favour of MAT+ASCT over conventional chemotherapy (HR = 0.79, 95% CI 0.67–0.93, p = 0.004; Fig. 2).
Non-RCT comparative studies (Supplemental Table 5) mostly support MAT+ASCT, though some favour non-MAT+ASCT or show no effect. While the benefit was more pronounced in patients with complete response (CR)/very good partial response (VGPR) after induction in RCTs [10, 39, 40], some non-RCT studies report greater effects in patients with partial response (PR) [41]. However, bias inherent in the study design appears to have a considerable impact on results.
In one non-RCT study (Simon et al. [42]), HR for MAT+ASCT versus no MAT+ASCT were 0.64 for EFS and 0.67 for OS, and were statistically significant [42], although approximately half of the patients received anti-GD2 therapy in the maintenance setting. In the analysis of patients from this study not treated with anti-GD2 therapy, the benefit of MAT+ASCT was clearly pronounced, with EFS and OS almost doubling after 9 years [42]. Another study (Stram et al. [12]) with approximately 50% of patients in CR/VGPR reported doubling 4-year EFS with MAT+ASCT (40% versus 19%, p = 0.020) [12]. However, no effect of MAT+ASCT was found in infants [43]. Imaizumi et al. reported 7-year EFS approximately four-fold greater in the MAT+ASCT group; however, more than twice as many patients in this study had favourable response to induction in the ASCT versus non-ASCT group [44]. In another study (Yan et al. [45]) with over 90% of patients in CR/VGPR following induction, 3-year EFS was 24.2% greater in the MAT+ASCT group, while OS was 19.2% greater, although in the transplantation group 27.1% received a tandem procedure [45]. Even at 10 years, patients treated with MAT had a significantly better outcome, with OS 9% greater and EFS 6% greater than those receiving continuous chemotherapy (p = 007 and p = 0.0052, respectively; Hero et al. [41]). Indeed, patients with PR to induction chemotherapy appeared to gain from MAT, with OS almost doubled (0.23 versus 0.12, p = 0.0167) and EFS almost tripled (0.25 versus 0.09, p = 0.0031), while patients with CR did not benefit [41].
In a study with consecutive, unselected patients (Luksch et al. [46]), the 5-year EFS was 31% for MAT+ASCT versus 12% for comparator with irradiation (p = 0.03); the 5-year OS was nearly tripled at 35% versus 12% (p = 0.03). Approximately 40% of patients in this study achieved CR/VGPR after induction [46].
Additionally, MAT+ASCT was associated with significantly better 5-year OS in a large, comprehensive, nationwide registry-based study by Imaizumi et al. [44]. Univariate and multivariate analyses favoured MAT+ASCT (HR = 0.45, 95% CI 0.34–0.60, p < 0.01 and HR = 0.46, 95% CI 0.32–0.64, p < 0.01, respectively) [44]. As anti-GD2 immunotherapy was given to some patients, it was entered into the multivariate model so that HR was not biased by treatment modality [44].
Significant benefit of MAT+ASCT was found in a large international study analysing two different age subgroups of patients (Mosse et al. [47]). Among patients ≥ 5–< 10 years, those who received MAT+ASCT had significantly superior rates compared with those who did not, with both 5-year EFS (30 ± 6% versus 14 ± 3%, p = 0.0001) and OS (43 ± 6% versus 23 ± 3%, p = 0.001) rates doubled in the MAT+ASCT group. Similar magnitude of both EFS and OS benefit of MAT+ASCT was observed in patients ≥ 10 years [47].
The greatest benefit of MAT+ASCT was found in patients with incomplete resection of primary tumour at 10 years with disease-free survival more than three-fold greater than in the group not treated with MAT+ASCT (85% versus 25%, p = 0.015; Moon et al. [48]).
A small single-centre study (Chamberlain et al. [49]) found no significant difference between MAT+ASCT and chemotherapy for PFS and OS at approximately 2 years; however, a trend favouring MAT+ASCT was evident with PFS doubling at 18 months (25.8% versus 13.2%, p = 0.06). A non-significant trend for MAT+ASCT versus maintenance chemotherapy was found in a study with 47% of patients in CR/VGPR (Castel et al. [50]), where 5-year OS was doubled in favour of MAT+ASCT (56% versus 28%, p = non-significant).
A benefit for MAT+ASCT was reported by Ohnuma et al. separately for stage 4 and stage 3 patients [51]. For stage 4 patients, a 6% greater 5-year OS and 10% greater 5-year EFS in favour of MAT+ASCT was found, but the difference was not statistically significant [51]. For stage 3 advanced patients with neuroblastoma, both 2-year EFS and 5-year OS were approximately 70% greater after MAT+ASCT, with the difference being statistically significant [51].
Survival benefit might be subject to selection bias, particularly if only patients with better prognosis are selected for MAT+ASCT. Berthold et al. reported statistically significantly better EFS for MAT+ASCT versus chemotherapy; however, after adjustment for response to induction, the benefit was no longer significant [52]. Unadjusted OS was significantly better on MAT+ASCT, although the trend reversed after 4 years (0.18 versus 0.25, respectively) [52]. In this study, up to 40% of patients achieved CR after induction [52]. In another study by DeBernardi et al., a non-significant trend was seen among patients who received MAT for both OS (HR = 0.76, 95% CI 0.53–1.09) and EFS (HR = 0.71, 95% CI 0.51–1.01) after adjustment for response to induction with approximately 5% of patients having achieved CR and the majority PR [31]. A lack of OS benefit at 5 and 10 years was also reported in a study with two different chemotherapy regimens Kaneko et al. [53]. In this multicentre study, ASCT [bone marrow transplant (BMT)] was feasible only in selected centres, which may have caused selection bias [53]. Among the consolidated patients, CR was achieved post-induction in 81% of patients [53]. The same group (Kaneko et al. [54]) reported the effect of MAT + autologous BMT/peripheral blood stem cell transplantation (PBSCT) versus chemotherapy on RFS in subgroups based on response to induction and MYCN status [54]. In patients with PR/stable disease/progressive disease, PFS at 5 years was comparable; i.e., there was no benefit of MAT + autologous BMT/PBSCT [54]. In contrast, in patients with CR and MYCN amplification, MAT + autologous BMT/PBSCT doubled RFS at 5 years, while in patients with CR and without MYCN amplification, the improvement was 27% [54].
One study Castel et al. [30] reported a non-significant trend of 13% difference in EFS in favour of maintenance chemotherapy versus MAT+ASCT (0.46 versus 0.33), but the time point for this analysis was not reported. In this study, approximately 50% of patients were in CR/VGPR [30]. Another study Klaassen et al. [55] that included approximately 50% patients with CR/VGPR found no difference in survival after adjustment for survivor and selection bias when selecting only patients alive at the median time to transplantation and showing at least a PR to up-front therapy. Without adjustment, 5-year OS was 22% greater after MAT+ASCT versus without MAT+ASCT [55]. Significantly better 5-year EFS (by 12%) and OS (by 6%) on chemotherapy rather than on MAT+ASCT were reported in a large multicentre study Aksoylar et al. [56]. The authors acknowledge bias in the selection of patients for ASCT, as patients with higher risk of adverse outcomes may be selected for the more intensive therapy with MAT+ASCT [56]. Moreover, the unbalanced distribution of patients with MYCN-amplified tumours in the two groups may have impacted the outcome.
3.3 MAT+ASCT with Subsequent Anti-GD2 Therapy
Studies comparing the added benefit of MAT+ASCT if followed by anti-GD2 immunotherapy versus anti-GD2 immunotherapy alone without prior consolidation with MAT+ASCT are limited to four non-RCT studies.
In a study of patients in CR/VGPR treated with m3F8 anti-GD2 immunotherapy, 5-year PFS and OS were 12% higher with prior MAT+ASCT Cheung et al. [57]. However, patients who received MAT+ASCT also subsequently received maintenance with isotretinoin, which could have favoured this treatment group [57]. Furthermore, survivor bias could have improved outcomes in this group, as patients who would have died prior to MAT+ASCT and immunotherapy were not included (survival was reported from the time of initiation of immunotherapy) [57]. In a separate study with CR/VGPR patients treated with m3F8 anti-GD2 immunotherapy and only differing with respect to MAT+ASCT Kushner et al. [28], the difference in 5-year EFS was 14% in favour of MAT+ASCT [65% (95% CI 54–78) versus 51% (95% CI 42–62), p = 0.128], but it was not statistically significant, and no trend for 5-year OS was observed [76% (95% CI 66–88) versus 75% (95% CI 68–85), p = 0.975], likely owing to the efficacy of subsequent salvage therapies.
In a retrospective, single-centre study Mora et al. [58], 2-year EFS and OS were not significantly different for anti-GD2 treated patients who received ASCT versus those who did not, and this result was similar in patients with CR (2-year EFS: 65.5% versus 58.7%, p = 0.48; 2-year OS: 71.4% versus 85.4%, (p = 0.63 for ASCT and non-ASCT patients, respectively). Patients treated with anti-GD2 immunotherapy without ASCT had non-significant trends towards superior 3-year OS (90.2% versus 77.6%, HR = 1.10, 95% CI 0.20–6.01, p = 0.92) but lower 3-year EFS (50.3% versus 64.7%, p = 0.78) compared with patients who received ASCT (Mora et al. [59]).
Additionally, a German study Simon et al. [60] reported that, in the subgroup of patients treated with anti-GD2 therapy after ASCT, 5-year EFS improved by 14% and OS by 6.4%, and respective 9-year EFS by 9.6% and OS by 3.1%; however, statistical significance was not reported for these comparisons [60]. In this study, 79% of patients were in CR/VGPR [60].
3.4 Tandem versus Single MAT+ASCT
Post-hoc analyses from the one identified RCT (Park et al. [17]) confirm the benefit of tandem MAT+ASCT in patients subsequently treated with anti-GD2 immunotherapy versus single MAT+ASCT (3-year EFS: 73.3% versus 54.7%, p = 0.004; 3-year OS: 84.0% versus 73.5%, p = 0.04). These data suggest that a second transplant prior to the start of anti-GD2 immunotherapy may reduce the burden of disease and lead to improvements in EFS and OS [17]. The study also suggests that this benefit may not be evident in the subgroup of patients who did not receive anti-GD2 immunotherapy.
Data from non-RCT studies of tandem versus single MAT+ASCT are heterogenous and subject to bias. A large multicentre study (Sung et al. [61]) with the majority of patients in CR or VGPR found that 5-year EFS was approximately 20% greater in the tandem MAT+ASCT group than in the single MAT+ASCT group (HR = 0.16, p < 0.05). In patients without CR, 5-year EFS was over 50% greater (p < 0.05), suggesting that patients in VGPR or less are likely to benefit more from tandem versus single MAT+ASCT [61]. However, more than twice as many patients received total body irradiation (TBI) in the tandem versus single MAT+ASCT group, and given that EFS after the tandem procedure was greater in the TBI group than in the non-TBI group, this may have resulted in the overestimation of EFS in the tandem group. In another large retrospective multicentre study (Qayed et al. [62]), 4-year EFS for patients who received a tandem MAT+ASCT was 32% higher (p < 0.05). Tandem MAT+ASCT also improved 4-year OS by 26%, though this difference was not significant. Multivariate regression model analysis, controlling for the use of TBI and disease response at the end of induction, confirmed the benefit of tandem MAT+ASCT with EFS (HR = 0.47) and OS (HR = 0.47), both of which were statistically significant [62]. A small single-centre study in stage 3 patients (Suh et al. [63]) reported that, at 5 years in the single MAT+ASCT group, 75% remained event free, while for patients who underwent the tandem procedure, EFS was 100% [63].
In contrast, a retrospective study by Yan et al. [45] with a small subgroup of patients receiving MAT+ASCT found 3-year EFS to be 22% higher in the single versus tandem transplantation (51.9% versus 73.8%), while OS was 12% higher (71.4% versus 83.4%), although for both outcomes the trend was not significant (p = 0.44 and p = 0.73, respectively). These data suggest a lack of survival benefit for tandem ASCT if not followed by anti-GD2 immunotherapy. No benefit was also found in a retrospective, single-centre study (Kim et al. [64]) where at 2 years the disease-free survival and OS of patients who underwent double autologous PBSCT were not different from those of patients who underwent single autologous PBSCT [64]. Additionally, a prospective, multicentre study by Frappaz et al. [65] comparing immediate single MAT+ASCT versus double MAT+ASCT versus additional chemotherapy followed by single MAT+ASCT found that PFS at 7 years was approximately double for single MAT+ASCT. Statistical significance for the relevant comparison versus tandem MAT+ASCT was not tested, but PFS in the single MAT+ASCT group was significantly better compared with combined tandem MAT+ASCT and chemotherapy followed by single MAT+ASCT group.
3.5 Studies in Relapsed Patients
One retrospective analysis of three large prospective multicentre studies (Simon et al. [66]) compared MAT+ASCT after relapse with chemotherapy or no treatment [66]. In patients with MAT+ASCT, 3-year OS was 43.5% from recurrence while following second-line chemotherapy, but without second MAT+ASCT the 3-year survival rate from event was under 10%, with the difference being statistically significant [66]. However, the results are likely affected by survivorship bias, as survival in patients who received MAT+ASCT is reported from this procedure, i.e., no mortality between relapse and MAT+ASCT was reported, unlike in the chemotherapy group where mortality was also captured during induction chemotherapy and prior to MAT.
4 Discussion
This systematic review of 34 studies compared survival outcomes in patients with HRNB treated with or without single or tandem MAT+ASCT with or without subsequent anti-GD2 immunotherapy. The meta-analysis of the RCTs [10, 13, 38,39,40] provided evidence of statistically significant difference in EFS in favour of MAT+ASCT over conventional chemotherapy or no further treatment (HR = 0.78, 95% CI 0.67−0.91, p = 0.001) and a non-significant trend favouring MAT+ASCT for OS (HR = 0.86, 95% CI 0.73−1.00, p = 0.05). Our meta-analysis, which included long-term results from the GPOH NB97 study [40], confirms the previously reported Cochrane review findings with statistically significant EFS benefit (HR = 0.78, 95% CI 0.67–0.90, p = 0.0006) and a non-significant trend for OS benefit (HR = 0.86, 95% CI 0.73–1.01) [18]. The non-RCT comparative studies systematically reviewed demonstrate findings that are broadly consistent with evidence from the RCTs; however, not all non-RCT studies reported survival benefit for MAT+ASCT. Subgroup data from RCTs suggest greater benefit of MAT+ASCT in patients with CR/VGPR after induction, but no clear pattern has been observed in non-RCT studies. Conclusions related to MYCN status are limited to a subgroup analysis from one non-RCT, suggesting greater benefits in patients with MYCN-amplified tumours [54].
Limited comparative evidence exists for the survival benefit of MAT+ASCT followed by anti-GD2 immunotherapy versus anti-GD2 therapy alone without prior MAT+ASCT. Even so, it is advisable not to exclude MAT+ASCT from multimodal therapy to minimise relapse risk. However, in patients treated with anti-GD2 immunotherapy, prior tandem ASCT versus single ASCT appears to offer improvement in both EFS and OS [17].
It is unclear whether tandem MAT+ASCT offers additional survival benefits over the single procedure in patients who do not receive subsequent immunotherapy. One RCT demonstrated statistically significant improvement in EFS but no benefit for OS [17]. The EFS benefit was observed in the overall patient population, specifically in the subgroup treated with anti-GD2 immunotherapy. For the subgroup not treated with immunotherapy, results were not reported, but the magnitude of effect suggests that in these patients tandem SCT seemed to provide no additional advantages. It is unclear whether specific patient subgroups benefit more from tandem MAT+ASCT based on their response to induction chemotherapy. It also requires confirmation in patients who received different induction regimens. As stated in the introduction, the discussion on the benefit of MAT+ASCT was initiated during a SIOP workshop and by patient advocacy, but it is also most relevant to current practice. Some published analyses suggest that, with anti-GD2 immunotherapy, OS with and without MAT+ASCT might be comparable in some patient groups, and a different approach to the consolidation treatment has been proposed [28, 34]. It is hypothesized that treatment using anti-GD2 immunotherapy in consolidation after induction chemotherapy, omitting MAT+ASCT, may result in similar survival as following ASCT, which is the standard of care recommended by cooperative groups (e.g. SIOPEN, COG, etc.). However, these studies are based on two single-centre experiences [28, 58]. Kushner et al. [28] presented data of patients in first CR/VGPR treated with m3F8 plus GM-CSF and isotretinoin, with or without previous MAT+ASCT. The study was not randomised, and the choice of the treatment protocol was dependent on the treatment centre. The 5-year EFS and OS for ASCT-treated patients versus non-ASCT-treated patients were not significantly different [28]. According to the authors, longer time from first chemotherapy to therapy with m3F8 had no impact on EFS in a multivariate analysis (p = 0.156), so the postponed implementation of immunotherapy caused by recovery time after ASCT should not negatively influence survival [28]. The authors do not report on subsequent therapies, but comparable OS for both groups in the study may also be strongly influenced by the more effective treatment of relapses, given a time-consistent trend for increased risk of relapse without MAT+ASCT (5-year EFS: 51% versus 65%, p = 0.128) [67].
Similar conclusions were drawn by Mora et al. [58] with the use of different anti-GD2 antibodies (dinutuximab or naxitamab) as part of two different clinical studies or as a compassionate use programme, which included patients treated in first line who were either in CR or were refractory (i.e., had persistent disease, but only in bones or bone marrow with the exclusion of harder to treat soft tissue disease). There were no significant differences in 2-year EFS (64.1% versus 54.2%, p = 0.28) or OS (66.7% versus 84.1%, p = 0.81) for ASCT and non-ASCT patients, respectively [58]. Results may also be influenced by the number of immunotherapy cycles. In the COG and SIOPEN protocols, up to 5 cycles of anti-GD2 immunotherapy are given, whereas in Mora et al.’s study up to 10 immunotherapy cycles were allowed, although the difference in outcome was not statistically significant in patients receiving more and less than 5 cycles [58]. However, the number of patients in this study was relatively low, the group was heterogenous and no information concerning treatment after the potential relapses was provided, which could also strongly influence OS [58].
Although Mora et al. [58] included patients in CR as well as refractory patients, the study does not permit the drawing of conclusions on the efficacy of MAT+ASCT in the two populations. Specifically, as the published results provided no relevant evidence, no conclusions can be drawn on the effect of ASCT prior to naxitamab in primary refractory patients, i.e., in the population where this anti-GD2 immunotherapy is licensed in some countries [58].
Induction regimens varied widely between groups in this study (e.g., in naxitamab-treated patients, 66.7% received induction according to the Chinese CCCG NB2014 protocol), and generalisability of the results is hindered by the fact that the majority of patients (79.1%) received at least six cycles of induction chemotherapy; i.e., N7 or N7+CTV was used instead of currently preferred N5 [58]. N5 and N7 protocols are associated with comparable response rates [68], and there are no data comparing survival rates between the two regimens. If fewer numbers of induction cycles are less effective in preventing relapse, and if induction is not followed by MAT+ASCT to further control the disease, survival could be negatively impacted. However, it is known that adding further chemotherapy to some other known induction schedules does not influence survival [69, 70].
Mora et al. [59] evaluated patients in first or second CR (excluding therapy-resistant patients) treated with naxitamab plus GM-CSF as consolidation in a compassionate use programme. In all, 3-year EFS was 58.4% and OS was 82.4%, with EFS being significantly different between patients in first and second CR (p = 0.0029) [59]. However, OS was not influenced by first- or second-line therapy [59], which may show the potential influence of the subsequent treatment lines on OS using modern treatment methods.
Determination of effect of MAT in Mora et al. [59] requires separate analysis of newly diagnosed and relapsed patients, because relapsed patients with prior MAT may have received it either before or after relapse. Included patients showed a trend of worse EFS and OS post-ASCT (EFS: HR = 3.21, 95% CI 1.43–7.21, p = 0.0047; OS: HR = 2.88, 95% CI 0.58–14.4, p = 0.20), possibly owing to the fact that the ASCT was given mainly to newly diagnosed patients as consolidation prior to relapse, and relapse is associated with poorer prognosis. Even so, reported as a percentage, OS was 12.6% better in the post-ASCT subgroup, although the difference was not statistically significant [59]. Interpretation of these results is further complicated by lack of details on prior and subsequent treatments, particularly the type of MAT [59].
Omitting MAT+ASCT as an approach to therapy is not based on RCT evidence, but only on single-centre non-RCT studies. The justification is based on treatment modifications in comparison with previous studies, such as the use of anti-GD2 immunotherapy with GM-CSF [71] and use of local radiotherapy and isotretinoin in all patients. According to the authors, the improvement of treatment results with MAT+ASCT cannot be compared with modern treatment results, as historical treatments did not include maintenance treatment or consisted of oral cyclophosphamide without immunotherapy [38, 39].
In the GPOH NB97 study, the reported OS HR was statistically significant for patients with CR/VGPR [39]. Also, in Matthay et al. [10], the effect of MAT+ASCT on 5-year EFS was much greater in patients with CR/VGPR than those with PR/mixed response/stable disease (15% versus 9%) [10]. Long-term follow-up data from the NB97 study showed a statistically significant effect of MAT+SCT in the “as treated” population with CR/VGPR (10-year OS: HR = 1.65, 95% CI 1.05–2.61) [40]. Therefore, based on long-term evidence, it can be hypothesised that MAT+ASCT improves both EFS and OS in patients with CR/VGPR, though the benefit in patients with PR appears to be less pronounced.
Importantly, the studies included in the Cochrane review by Yalcin et al. [18] did not include patients treated with effective anti-GD2 immunotherapy. In the NB97 study, some patients were treated with anti-GD2 immunotherapy in maintenance. Although immunotherapy was not a predictor of efficacy [40], the analysis of long-term results showed improvement in OS in patients treated with MAT followed by immunotherapy in comparison with other groups treated without MAT [60]. The anti-GD2 antibody in the study was not licensed for use and was administered in cycles every 2 months, a regimen which most likely did not control minimal residual disease [40]. The NB97 study showed that, with anti-GD2 immunotherapy with no proven efficacy (which would be equivalent to treatment without anti-GD2 therapy), MAT+ASCT improves EFS and OS in patients with CR/VGPR [40]. With no controlled survival evidence for naxitamab in patients receiving it without prior MAT, omitting MAT+ASCT before naxitamab immunotherapy for PR/VGPR/CR patients might lower survival prospects in this group. There is also no comparative evidence at present for treatment with and without MAT+ASCT followed by any anti-GD2 immunotherapy in the refractory setting, and the only evidence for patients in CR/VGPR comes from a single-centre study with an unlicensed antibody [28]. Importantly, it needs to be highlighted that evidence from patients treated with one particular anti-GD2 immunotherapy should not be used to infer efficacy of another, and only immunotherapies with demonstrable comparative efficacy should be considered in the context of MAT+ASCT, as for some, such comparative benefit for survival had not been demonstrated (e.g. in the GPOH NB97 trial [40] and a compassionate use programme with naxitamab as consolidation for patients with HRNB in first CR [72]).
At this time, the identified evidence does not support any differentiating claims that one particular anti-GD2 immunotherapy can be used without prior MAT+ASCT while other anti-GD2 therapies cannot. The only evidence for the added benefit of MAT+ASCT prior to immunotherapy is available for m3F8 (unlicensed murine antibody) and only in a subgroup of patients with CR/VGPR [28]. This evidence shows lack of effect on OS [76% (95% CI 66–88) versus 76% (95% CI 68–85), p = 0.975] and a non-significant, although clear, trend for EFS [65% (95% CI 54–78) versus 51% (95% CI 42–62), p = 0.128] [28]. Importantly, it cannot be assumed that the same would apply to the humanised antibody 3F8 [naxitamab (hu3F8)] or chimeric antibodies (dinutuximab and dinutuximab beta). As there are no comparative head-to-head studies of different antibodies, we can only speculate that the evidence for one of them may apply to the others.
The only biological factor analysed in most reviewed studies is MYCN amplification, and its influence on survival differs among studies—varying from worse outcomes [53] to better outcomes [12]. It is now known that, in addition to MYCN amplification, there are other genetic factors strongly influencing survival. Their presence may also cause either rapid occurrence of relapses, or long course of the disease, with very poor outcomes reported after a long observation period. The influence of these genetic factors also needs to be taken into consideration, as different genetic risk groups may or may not benefit from MAT+ASCT [73,74,75].
4.1 Limitations
The main limitation of the analysis is the heterogenous and historical nature of the therapies used, including different induction and consolidation schedules, different approaches to radiotherapy (including TBI) and use of isotretinoin. Different induction schedules may influence the response to MAT+ASCT and its toxicities, which could in turn affect OS outcomes, as the efficacy of a regimen might depend on prior, concomitant and subsequent treatment. Another limitation is that baseline characteristics are often not reported in detail, and response to treatment is not reported consistently and is based on changing criteria. Additionally, the definition of the high-risk group is not consistent among studies, especially inclusion of infants and localised tumours with MYCN amplification or unfavourable biology.
Unfortunately, immunotherapy with proven comparative efficacy was not used in studies directly comparing MAT+ASCT with maintenance treatment or no treatment [39]. Additional factors that may have influenced the comparison of studies conducted over a long time include the experience of centres in performing the ASCT procedure, the source of cells (BMT or PBSCT), and recent improvements of supportive treatment in paediatric oncology and transplantology. All these factors may strongly influence response to therapy and survival. PBSCT rather than BMT is now the procedure of choice due to the more rapid recovery after the procedure, easier and more efficient collection of cells [76] and probably less contamination with neuroblastoma cells [77].
5 Conclusions
The evidence supports the use of MAT+ASCT for reducing the risk of relapse in patients with HRNB. While the OS benefit trend lacks significance, possibly due to post-relapse treatments, limited evidence suggests poorer PFS/EFS survival outcomes when MAT+ASCT is omitted prior to anti-GD2 immunotherapy. Owing to the high morbidity associated with relapse and toxicity of salvage treatment, MAT+ASCT should remain an integral part of multimodal therapy until further evidence emerges. Early findings hint at sufficient efficacy of anti-GD2 immunotherapy without MAT+ASCT in some patient groups, suggesting that omitting MAT+ASCT may be suitable when its administration is not feasible owing to toxicity or economic reasons in countries where reimbursement is limited. Evidence also supports tandem MAT+ASCT, especially when followed by anti-GD2 therapy. Limited evidence suggests better survival with MAT+ASCT administered post-relapse, and emerging chemoimmunotherapy may enhance outcomes. Future research, ideally using RCTs if feasible, may identify patient groups that do not need intensification therapy, such as tandem MAT+ASCT, or those benefiting from earlier anti-GD2 immunotherapy.
References
Swift CC, Eklund MJ, Kraveka JM, Alazraki AL. Updates in diagnosis, management, and treatment of neuroblastoma. Radiographics. 2018;38:566–80. https://doi.org/10.1148/rg.2018170132.
Whittle SB, Smith V, Doherty E, Zhao S, McCarty S, Zage PE. Overview and recent advances in the treatment of neuroblastoma. Expert Rev Anticancer Ther. 2017;17:369–86. https://doi.org/10.1080/14737140.2017.1285230.
Smith MA, Seibel NL, Altekruse SF, Ries LA, Melbert DL, O’Leary M, et al. Outcomes for children and adolescents with cancer: challenges for the twenty-first century. J Clin Oncol. 2010;28:2625–34. https://doi.org/10.1200/jco.2009.27.0421.
Park JR, Eggert A, Caron H. Neuroblastoma: biology, prognosis, and treatment. Hematol Oncol Clin North Am. 2010;24:65–86. https://doi.org/10.1016/j.hoc.2009.11.011.
DuBois SG, Macy ME, Henderson TO. High-risk and relapsed neuroblastoma: toward more cures and better outcomes. Am Soc Clin Oncol Educ Book. 2022;42:1–13. https://doi.org/10.1200/EDBK_349783.
Ladenstein R, Potschger U, Valteau-Couanet D, Luksch R, Castel V, Ash S, et al. Investigation of the role of dinutuximab beta-based immunotherapy in the SIOPEN High-Risk Neuroblastoma 1 Trial (HR-NBL1). Cancers (Basel). 2020;12(2):309. https://doi.org/10.3390/cancers12020309.
Bellini A, Pötschger U, Bernard V, Lapouble E, Baulande S, Ambros PF, et al. Frequency and prognostic impact of alk amplifications and mutations in the European Neuroblastoma Study Group (SIOPEN) High-Risk Neuroblastoma Trial (HR-NBL1). J Clin Oncol. 2021;39:3377–90. https://doi.org/10.1200/jco.21.00086.
Hogarty MD, Brodeur GM. Chapter 457. Neuroblastoma. In: Rudolph CD, Rudolph AM, Lister GE, First LR, Gershon AA, editors. Rudolph’s Pediatrics. 22nd ed. New York: The McGraw-Hill Companies; 2011.
Smith V, Foster J. High-risk neuroblastoma treatment review. Children (Basel). 2018;5(9):114. https://doi.org/10.3390/children5090114.
Matthay KK, Reynolds CP, Seeger RC, Shimada H, Adkins ES, Haas-Kogan D, et al. Long-term results for children with high-risk neuroblastoma treated on a randomized trial of myeloablative therapy followed by 13-cis-retinoic acid: a children’s oncology group study. J Clin Oncol. 2009;27:1007–13. https://doi.org/10.1200/JCO.2007.13.8925.
Fish JD, Grupp SA. Stem cell transplantation for neuroblastoma. Bone Marrow Transplant. 2008;41:159–65. https://doi.org/10.1038/sj.bmt.1705929.
Stram DO, Matthay KK, O’Leary M, Reynolds CP, Haase GM, Atkinson JB, et al. Consolidation chemoradiotherapy and autologous bone marrow transplantation versus continued chemotherapy for metastatic neuroblastoma: a report of two concurrent Children’s Cancer Group studies. J Clin Oncol. 1996;14:2417–26. https://doi.org/10.1200/JCO.1996.14.9.2417.
Matthay KK, Villablanca JG, Seeger RC, Stram DO, Harris RE, Ramsay NK, et al. Treatment of high-risk neuroblastoma with intensive chemotherapy, radiotherapy, autologous bone marrow transplantation, and 13-cis-retinoic acid. Children’s Cancer Group. N Engl J Med. 1999;341:1165–73. https://doi.org/10.1056/NEJM199910143411601.
Yu AL, Gilman AL, Ozkaynak MF, London WB, Kreissman SG, Chen HX, et al. Anti-GD2 antibody with GM-CSF, interleukin-2, and isotretinoin for neuroblastoma. N Engl J Med. 2010;363:1324–34. https://doi.org/10.1056/NEJMoa0911123.
Ladenstein R, Potschger U, Valteau-Couanet D, Luksch R, Castel V, Yaniv I, et al. Interleukin 2 with anti-GD2 antibody ch14.18/CHO (dinutuximab beta) in patients with high-risk neuroblastoma (HR-NBL1/SIOPEN): a multicentre, randomised, phase 3 trial. Lancet Oncol. 2018;19:1617–29. https://doi.org/10.1016/S1470-2045(18)30578-3.
Ladenstein R, Potschger U, Pearson ADJ, Brock P, Luksch R, Castel V, et al. Busulfan and melphalan versus carboplatin, etoposide, and melphalan as high-dose chemotherapy for high-risk neuroblastoma (HR-NBL1/SIOPEN): an international, randomised, multi-arm, open-label, phase 3 trial. Lancet Oncol. 2017;18:500–14. https://doi.org/10.1016/S1470-2045(17)30070-0.
Park JR, Kreissman SG, London WB, Naranjo A, Cohn SL, Hogarty MD, et al. Effect of tandem autologous stem cell transplant vs single transplant on event-free survival in patients with high-risk neuroblastoma: a randomized clinical trial. JAMA. 2019;322:746–55. https://doi.org/10.1001/jama.2019.11642.
Yalcin B, Kremer LC, van Dalen EC. High-dose chemotherapy and autologous haematopoietic stem cell rescue for children with high-risk neuroblastoma. Cochrane Database Syst Rev. 2015;2015: CD006301. https://doi.org/10.1002/14651858.CD006301.pub4.
Martin A, Schneiderman J, Helenowski IB, Morgan E, Dilley K, Danner-Koptik K, et al. Secondary malignant neoplasms after high-dose chemotherapy and autologous stem cell rescue for high-risk neuroblastoma. Pediatr Blood Cancer. 2014;61:1350–6. https://doi.org/10.1002/pbc.25033.
Applebaum MA, Vaksman Z, Lee SM, Hungate EA, Henderson TO, London WB, et al. Neuroblastoma survivors are at increased risk for second malignancies: a report from the International Neuroblastoma Risk Group Project. Eur J Cancer. 2017;72:177–85. https://doi.org/10.1016/j.ejca.2016.11.022.
Elzembely MM, Dahlberg AE, Pinto N, Leger KJ, Chow EJ, Park JR, et al. Late effects in high-risk neuroblastoma survivors treated with high-dose chemotherapy and stem cell rescue. Pediatr Blood Cancer. 2019;66: e27421. https://doi.org/10.1002/pbc.27421.
Friedman DN, Henderson TO. Late effects and survivorship issues in patients with neuroblastoma. Children (Basel). 2018;5(8):107. https://doi.org/10.3390/children5080107.
Moke DJ, Luo C, Millstein J, Knight KR, Rassekh SR, Brooks B, et al. Prevalence and risk factors for cisplatin-induced hearing loss in children, adolescents, and young adults: a multi-institutional North American cohort study. Lancet Child Adolesc Health. 2021;5:274–83. https://doi.org/10.1016/s2352-4642(21)00020-1.
Spears N, Lopes F, Stefansdottir A, Rossi V, De Felici M, Anderson RA, et al. Ovarian damage from chemotherapy and current approaches to its protection. Hum Reprod Update. 2019;25:673–93. https://doi.org/10.1093/humupd/dmz027.
Laverdière C, Cheung NK, Kushner BH, Kramer K, Modak S, LaQuaglia MP, et al. Long-term complications in survivors of advanced stage neuroblastoma. Pediatr Blood Cancer. 2005;45:324–32. https://doi.org/10.1002/pbc.20331.
Schechter T, Perez-Albuerne E, Lin TF, Irwin MS, Essa M, Desai AV, et al. Veno-occlusive disease after high-dose busulfan-melphalan in neuroblastoma. Bone Marrow Transplant. 2020;55:531–7. https://doi.org/10.1038/s41409-018-0298-y.
Niederwieser D, Baldomero H, Bazuaye N, Bupp C, Chaudhri N, Corbacioglu S, et al. One and a half million hematopoietic stem cell transplants: continuous and differential improvement in worldwide access with the use of non-identical family donors. Haematologica. 2022;107:1045–53. https://doi.org/10.3324/haematol.2021.279189.
Kushner BH, Ostrovnaya I, Cheung IY, Kuk D, Modak S, Kramer K, et al. Lack of survival advantage with autologous stem-cell transplantation in high-risk neuroblastoma consolidated by anti-GD2 immunotherapy and isotretinoin. Oncotarget. 2016;7:4155–66. https://doi.org/10.18632/oncotarget.6393.
Kushner BH, Modak S, Kramer K, Basu EM, Iglesias-Cardenas F, Roberts SS, et al. Immunotherapy with anti-G(D2) monoclonal antibody in infants with high-risk neuroblastoma. Int J Cancer. 2023;152:259–66. https://doi.org/10.1002/ijc.34233.
Castel V, Canete A, Navarro S, Garcia-Miguel P, Melero C, Acha T, et al. Outcome of high-risk neuroblastoma using a dose intensity approach: improvement in initial but not in long-term results. Med Pediatr Oncol. 2001;37:537–42. https://doi.org/10.1002/mpo.1248.
De Bernardi B, Nicolas B, Boni L, Indolfi P, Carli M, Cordero Di Montezemolo L, et al. Disseminated neuroblastoma in children older than one year at diagnosis: comparable results with three consecutive high-dose protocols adopted by the Italian Co-Operative Group for Neuroblastoma. J Clin Oncol. 2003;21:1592–601. https://doi.org/10.1200/JCO.2003.05.191.
Mody R, Yu AL, Naranjo A, Zhang FF, London WB, Shulkin BL, et al. Irinotecan, temozolomide, and dinutuximab with GM-CSF in children with refractory or relapsed neuroblastoma: a report from the Children’s Oncology Group. J Clin Oncol. 2020;38:2160–9. https://doi.org/10.1200/jco.20.00203.
Federico SM, McCarville MB, Shulkin BL, Sondel PM, Hank JA, Hutson P, et al. A pilot trial of humanized anti-GD2 monoclonal antibody (hu14.18K322A) with chemotherapy and natural killer cells in children with recurrent/refractory neuroblastoma. Clin Cancer Res. 2017;23:6441–9. https://doi.org/10.1158/1078-0432.Ccr-17-0379.
Mora J. Autologous stem-cell transplantation for high-risk neuroblastoma: historical and critical review. Cancers (Basel). 2022;14(11):2572. https://doi.org/10.3390/cancers14112572.
Kushner BH. Re-thinking transplant for neuroblastoma. Pediatr Blood Cancer. 2021;68: e28961. https://doi.org/10.1002/pbc.28961.
Bird N, Scobie N, Palmer A, Ludwinski D. To transplant, or not to transplant? That is the question. A patient advocate evaluation of autologous stem cell transplant in neuroblastoma. Pediatr Blood Cancer. 2022;69: e29663. https://doi.org/10.1002/pbc.29663.
Flaadt T, Ladenstein RL, Ebinger M, Lode HN, Arnardóttir HB, Poetschger U, et al. Anti-GD2 Antibody dinutuximab beta and low-dose interleukin 2 after haploidentical stem-cell transplantation in patients with relapsed neuroblastoma: a multicenter, phase I/II trial. J Clin Oncol. 2023;41:3135–48. https://doi.org/10.1200/jco.22.01630.
Pritchard J, Cotterill SJ, Germond SM, Imeson J, de Kraker J, Jones DR. High dose melphalan in the treatment of advanced neuroblastoma: results of a randomised trial (ENSG-1) by the European Neuroblastoma Study Group. Pediatr Blood Cancer. 2005;44:348–57. https://doi.org/10.1002/pbc.20219.
Berthold F, Boos J, Burdach S, Erttmann R, Henze G, Hermann J, et al. Myeloablative megatherapy with autologous stem-cell rescue versus oral maintenance chemotherapy as consolidation treatment in patients with high-risk neuroblastoma: a randomised controlled trial. Lancet Oncol. 2005;6:649–58. https://doi.org/10.1016/s1470-2045(05)70291-6.
Berthold F, Ernst A, Hero B, Klingebiel T, Kremens B, Schilling FH, et al. Long-term outcomes of the GPOH NB97 trial for children with high-risk neuroblastoma comparing high-dose chemotherapy with autologous stem cell transplantation and oral chemotherapy as consolidation. Br J Cancer. 2018;119:282–90. https://doi.org/10.1038/s41416-018-0169-8.
Hero B, Kremens B, Klingebiel T, Bender-Götze C, Burdach S, Schrappe M, et al. Does megatherapy contribute to survival in metastatic neuroblastoma? A retrospective analysis. German Cooperative Neuroblastoma Study Group. Klin Padiatr. 1997;209:196–200. https://doi.org/10.1055/s-2008-1043950.
Simon T, Hero B, Faldum A, Handgretinger R, Schrappe M, Niethammer D, et al. Consolidation treatment with chimeric anti-GD2-antibody ch14.18 in children older than 1 year with metastatic neuroblastoma. J Clin Oncol. 2004;22:3549–57. https://doi.org/10.1200/jco.2004.08.143.
Simon T, Hero B, Faldum A, Handgretinger R, Schrappe M, Niethammer D, et al. Infants with stage 4 neuroblastoma: the impact of the chimeric anti-GD2-antibody ch14.18 consolidation therapy. Klin Padiatr. 2005;217:147–52. https://doi.org/10.1055/s-2005-836518.
Imaizumi M, Watanabe A, Kikuta A, Takano T, Ito E, Shimizu T, et al. Improved survival of children with advanced neuroblastoma treated by intensified therapy including myeloablative chemotherapy with stem cell transplantation: a retrospective analysis from the Tohoku Neuroblastoma Study Group. Tohoku J Exp Med. 2001;195:73–83. https://doi.org/10.1620/tjem.195.73.
Yan J, Jie L, Jiaxing Y, Yanna C, Zhanglin L, Zhongyuan L, et al. Analysis of the efficacy of autologous peripheral blood stem cell transplantation in high-risk neuroblastoma. Int J Med Sci. 2022;19:1715–23. https://doi.org/10.7150/ijms.76305.
Luksch R, Podda M, Gandola L, Polastri D, Piva L, Castellani R, et al. Stage 4 neuroblastoma: sequential hemi-body irradiation or high-dose chemotherapy plus autologous haemopoietic stem cell transplantation to consolidate primary treatment. Br J Cancer. 2005;92:1984–8. https://doi.org/10.1038/sj.bjc.6602615.
Mossé YP, Deyell RJ, Berthold F, Nagakawara A, Ambros PF, Monclair T, et al. Neuroblastoma in older children, adolescents and young adults: a report from the International Neuroblastoma Risk Group project. Pediatr Blood Cancer. 2014;61:627–35. https://doi.org/10.1002/pbc.24777.
Moon SB, Park KW, Jung SE, Youn WJ. Neuroblastoma: treatment outcome after incomplete resection of primary tumors. Pediatr Surg Int. 2009;25:789–93. https://doi.org/10.1007/s00383-009-2417-8.
Chamberlain RS, Quinones R, Dinndorf P, Movassaghi N, Goodstein M, Newman K. Complete surgical resection combined with aggressive adjuvant chemotherapy and bone marrow transplantation prolongs survival in children with advanced neuroblastoma. Ann Surg Oncol. 1995;2:93–100. https://doi.org/10.1007/bf02303622.
Castel V, García-Miguel P, Melero C, Navajas A, Navarro S, Molina J, et al. The treatment of advanced neuroblastoma. Results of the Spanish Neuroblastoma Study Group (SNSG) studies. Eur J Cancer. 1995;31a:642–5. https://doi.org/10.1016/0959-8049(95)00072-q.
Ohnuma N, Takahashi H, Kaneko M, Uchino J, Takeda T, Iwafuchi, et al. Treatment combined with bone marrow transplantation for advanced neuroblastoma: an analysis of patients who were pretreated intensively with the protocol of the Study Group of Japan. Med Pediatr Oncol. 1995;24:181–7. https://doi.org/10.1002/mpo.2950240308.
Berthold F, Burdach S, Kremens B, Lampert F, Niethammer D, Riehm H, et al. The role of chemotherapy in the treatment of children with neuroblastoma stage IV: the GPO (German Pediatric Oncology Society) experience. Klin Padiatr. 1990;202:262–9. https://doi.org/10.1055/s-2007-1025531.
Kaneko M, Tsuchida Y, Uchino J, Takeda T, Iwafuchi M, Ohnuma N, et al. Treatment results of advanced neuroblastoma with the first Japanese study group protocol. Study Group of Japan for Treatment of Advanced Neuroblastoma. J Pediatr Hematol Oncol. 1999;21:190–7. https://doi.org/10.1097/00043426-199905000-00006.
Kaneko M, Tsuchida Y, Mugishima H, Ohnuma N, Yamamoto K, Kawa K, et al. Intensified chemotherapy increases the survival rates in patients with stage 4 neuroblastoma with MYCN amplification. J Pediatr Hematol Oncol. 2002;24:613–21. https://doi.org/10.1097/00043426-200211000-00004.
Klaassen RJ, Trebo MM, Koplewitz BZ, Weitzman SS, Calderwood S. High-risk neuroblastoma in Ontario: a report of experience from 1989 to 1995. J Pediatr Hematol Oncol. 2003;25:8–13. https://doi.org/10.1097/00043426-200301000-00004.
Aksoylar S, Varan A, Vergin C, Hazar V, Akici F, Dagdemir A, et al. Treatment of high-risk neuroblastoma: national protocol results of the Turkish Pediatric Oncology Group. J Cancer Res Ther. 2017;13:284–90. https://doi.org/10.4103/0973-1482.183205.
Cheung NK, Cheung IY, Kushner BH, Ostrovnaya I, Chamberlain E, Kramer K, et al. Murine anti-GD2 monoclonal antibody 3F8 combined with granulocyte-macrophage colony-stimulating factor and 13-cis-retinoic acid in high-risk patients with stage 4 neuroblastoma in first remission. J Clin Oncol. 2012;30:3264–70. https://doi.org/10.1200/jco.2011.41.3807.
Mora J, Castañeda A, Flores MA, Santa-María V, Garraus M, Gorostegui M, et al. The role of autologous stem-cell transplantation in high-risk neuroblastoma consolidated by anti-GD2 immunotherapy. Results of two consecutive studies. Front Pharmacol. 2020;11: 575009. https://doi.org/10.3389/fphar.2020.575009.
Mora J, Castañeda A, Gorostegui M, Santa-María V, Garraus M, Muñoz JP, et al. Naxitamab combined with granulocyte-macrophage colony-stimulating factor as consolidation for high-risk neuroblastoma patients in complete remission. Pediatr Blood Cancer. 2021;68: e29121. https://doi.org/10.1002/pbc.29121.
Simon T, Hero B, Faldum A, Handgretinger R, Schrappe M, Klingebiel T, et al. Long term outcome of high-risk neuroblastoma patients after immunotherapy with antibody ch14.18 or oral metronomic chemotherapy. BMC Cancer. 2011;11:21. https://doi.org/10.1186/1471-2407-11-21.
Sung KW, Ahn HS, Cho B, Choi YM, Chung NG, Hwang TJ, et al. Efficacy of tandem high-dose chemotherapy and autologous stem cell rescue in patients over 1 year of age with stage 4 neuroblastoma: the Korean Society of Pediatric Hematology-Oncology experience over 6 years (2000–2005). J Korean Med Sci. 2010;25:691–7. https://doi.org/10.3346/jkms.2010.25.5.691.
Qayed M, Chiang KY, Ricketts R, Alazraki A, Tahvildari A, Haight A, et al. Tandem stem cell rescue as consolidation therapy for high-risk neuroblastoma. Pediatr Blood Cancer. 2012;58:448–52. https://doi.org/10.1002/pbc.23155.
Suh JM, Yoo KH, Sung KW, Kim JY, Cho EJ, Koo HH, et al. High-dose chemotherapy and autologous stem cell rescue in patients with high-risk stage 3 neuroblastoma: 10-year experience at a single center. J Korean Med Sci. 2009;24:660–7. https://doi.org/10.3346/jkms.2009.24.4.660.
Kim EK, Kang HJ, Park JA, Choi HS, Shin HY, Ahn HS. Retrospective analysis of peripheral blood stem cell transplantation for the treatment of high-risk neuroblastoma. J Korean Med Sci. 2007;22(Suppl):S66-72. https://doi.org/10.3346/jkms.2007.22.S.S66.
Frappaz D, Michon J, Coze C, Berger C, Plouvier E, Lasset C, et al. LMCE3 treatment strategy: results in 99 consecutively diagnosed stage 4 neuroblastomas in children older than 1 year at diagnosis. J Clin Oncol. 2000;18:468–76. https://doi.org/10.1200/jco.2000.18.3.468.
Simon T, Berthold F, Borkhardt A, Kremens B, De Carolis B, Hero B. Treatment and outcomes of patients with relapsed, high-risk neuroblastoma: results of German trials. Pediatr Blood Cancer. 2011;56:578–83. https://doi.org/10.1002/pbc.22693.
Lerman B, Li Y, Carlowicz C, Granger M, Cash T, Sadanand A, et al. Progression-free survival and patterns of response in patients with relapsed high-risk neuroblastoma treated with irinotecan/temozolomide/dinutuximab/GM-CSF. J Clin Oncol. 2023;41:508–16. https://doi.org/10.1200/JCO.22.01273.
Kushner BH, Kramer K, LaQuaglia MP, Modak S, Yataghene K, Cheung NK. Reduction from seven to five cycles of intensive induction chemotherapy in children with high-risk neuroblastoma. J Clin Oncol. 2004;22:4888–92. https://doi.org/10.1200/jco.2004.02.101.
Berthold F, Faldum A, Ernst A, Boos J, Dilloo D, Eggert A, et al. Extended induction chemotherapy does not improve the outcome for high-risk neuroblastoma patients: results of the randomized open-label GPOH trial NB2004-HR. Ann Oncol. 2020;31:422–9. https://doi.org/10.1016/j.annonc.2019.11.011.
Garaventa A, Poetschger U, Valteau-Couanet D, Luksch R, Castel V, Elliott M, et al. Randomized trial of two induction therapy regimens for high-risk neuroblastoma: HR-NBL1.5 International Society of Pediatric Oncology European Neuroblastoma Group Study. J Clin Oncol. 2021;39:2552–63. https://doi.org/10.1200/jco.20.03144.
Kushner BH, Kramer K, Cheung NK. Phase II trial of the anti-G(D2) monoclonal antibody 3F8 and granulocyte-macrophage colony-stimulating factor for neuroblastoma. J Clin Oncol. 2001;19:4189–94. https://doi.org/10.1200/jco.2001.19.22.4189.
Mora J, Castañeda A, Gorostegui M, Varo A, Perez-Jaume S, Simao M, et al. Naxitamab combined with granulocyte-macrophage colony-stimulating factor as consolidation for high-risk neuroblastoma patients in first complete remission under compassionate use-updated outcome report. Cancers (Basel). 2023;15(9):2535. https://doi.org/10.3390/cancers15092535.
Eleveld TF, Vernooij L, Schild L, Koopmans B, Alles LK, Ebus ME, et al. MEK inhibition causes BIM stabilization and increased sensitivity to BCL-2 family member inhibitors in RAS-MAPK-mutated neuroblastoma. Front Oncol. 2023;13:1130034. https://doi.org/10.3389/fonc.2023.1130034.
Koneru B, Farooqi A, Nguyen TH, Chen WH, Hindle A, Eslinger C, et al. ALT neuroblastoma chemoresistance due to telomere dysfunction-induced ATM activation is reversible with ATM inhibitor AZD0156. Sci Transl Med. 2021;13(607). https://doi.org/10.1126/scitranslmed.abd5750.
Valentijn LJ, Koster J, Zwijnenburg DA, Hasselt NE, van Sluis P, Volckmann R, et al. TERT rearrangements are frequent in neuroblastoma and identify aggressive tumors. Nat Genet. 2015;47:1411–4. https://doi.org/10.1038/ng.3438.
Hartmann O, Le Corroller AG, Blaise D, Michon J, Philip I, Norol F, et al. Peripheral blood stem cell and bone marrow transplantation for solid tumors and lymphomas: hematologic recovery and costs. A randomized, controlled trial. Ann Intern Med. 1997;126:600–7. https://doi.org/10.7326/0003-4819-126-8-199704150-00002.
Eguchi H, Takaue Y, Kawano Y, Watanabe A, Watanabe T, Kikuta A, et al. Peripheral blood stem cell autografts for the treatment of children over 1 year old with stage IV neuroblastoma: a long-term follow-up. Bone Marrow Transplant. 1998;21:1011–4. https://doi.org/10.1038/sj.bmt.1701207.
Schmidt ML, Lal A, Seeger RC, Maris JM, Shimada H, O’Leary M, et al. Favorable prognosis for patients 12 to 18 months of age with stage 4 nonamplified MYCN neuroblastoma: a Children’s Cancer Group Study. J Clin Oncol. 2005;23:6474–80. https://doi.org/10.1200/jco.2005.05.183.
Acknowledgements
The authors thank Kinga Krawczyk and Katarzyna Śladowska from Centrum HTA for data extraction and assessment support. Editorial assistance for the development of the manuscript was provided by mXm Medical Communications.
Funding
EUSA Pharma provided funding to Centrum HTA for data extraction and assessment support and to mXm Medical Communications for editorial support.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of Interest
UZ and WB received travel grants from EUSA Pharma; AW provided expert opinion to EUSA Pharma. JW is an employee of EUSA Pharma.
Ethical Approval
Not applicable.
Consent to Participate
Not applicable.
Consent for Publication
Not applicable.
Availability of Data and Material
Data and materials are available upon request from the corresponding author.
Code Availability
Not applicable.
Author Contributions
JW and AW conceived and designed the analysis; AW supervised the project; AW, UZ and JW collected the data; UZ and JW extracted the data; UZ, JW and AW contributed data or analysis tools; UZ, JW and AW performed the analysis; and UZ, JW, AW and WB wrote the manuscript. All authors reviewed and approved the final version of the manuscript.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution-NonCommercial 4.0 International License, which permits any non-commercial use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by-nc/4.0/.
About this article
Cite this article
Żebrowska, U., Balwierz, W., Wechowski, J. et al. Survival Benefit of Myeloablative Therapy with Autologous Stem Cell Transplantation in High-Risk Neuroblastoma: A Systematic Literature Review. Targ Oncol 19, 143–159 (2024). https://doi.org/10.1007/s11523-024-01033-4
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11523-024-01033-4