Abstract
This is a summary of a research article reporting Part A of the CheckMate 914 study (NCT03138512; EudraCT 2016-004502-34). Following surgery to remove renal cell carcinoma (RCC), people with a high risk of the cancer returning received nivolumab plus ipilimumab (adjuvant therapy) or placebo to see if this risk was reduced. The results of this study showed that the risk of RCC returning or death was not changed with adjuvant nivolumab plus ipilimumab treatment compared with placebo. In addition, people treated with nivolumab plus ipilimumab had more side effects compared with people treated with placebo (89% versus 57%).
Similar content being viewed by others
Avoid common mistakes on your manuscript.
1 Introduction
This summary of research article summarizes Part A of the CheckMate 914 study [1].
2 What is Renal Cell Carcinoma?
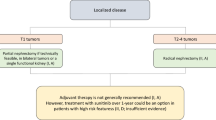
Renal cell carcinoma (RCC) is a type of cancer that begins in the lining of the nephrons in the kidney. Surgery is the standard treatment for people with early stages of RCC that is still within or next to the kidney and has not spread to elsewhere in the body. This surgery involves removing part of a kidney or kidney tumor (partial nephrectomy) or an entire kidney plus nearby tissue (radical nephrectomy). In about one-third of people who have surgery, RCC will come back within their kidneys or spread to other parts of the body.
3 What did Part A of this Study Look at?
In Part A of the CheckMate 914 study (NCT03138512; EudraCT 2016-004502-34) people at high risk of RCC coming back after partial or radical nephrectomy received nivolumab plus ipilimumab (adjuvant therapy) to see if it lowered the risk of RCC returning compared with the risk of disease returning in similar patients receiving placebo.
4 What were Researchers Investigating?
Researchers compared the median length of time people who received adjuvant nivolumab and ipilimumab survived and were free of the cancer returning after starting treatment (disease-free survival), with the median duration of disease-free survival in similar patients who received placebo after surgery. They also looked for side effects that people had with treatment.
5 Who Participated in Part A of this Study?
In total, 816 people from 20 countries participated: 405 people were assigned to receive treatment with nivolumab (one 240-mg dose every 2 weeks for a total of 12 doses) plus ipilimumab (one 1-mg/kg dose every 6 weeks for a total of 4 doses), and 411 people were assigned to receive placebo administered in the same way as the active treatment.
6 What did the Results of Part A of the Study Show?
The percentage of people who were living and cancer free at 24 months was 76% in the nivolumab plus ipilimumab group and 74% in the placebo group. Median disease-free survival was 50.7 months with placebo. Median disease-free survival with nivolumab plus ipilimumab could not be calculated because more than half of people assigned to this treatment were still cancer-free and living at the end of follow-up period (median follow-up was 37 months). The risk of RCC returning or death was not changed with adjuvant nivolumab plus ipilimumab treatment compared with placebo.
In people treated with nivolumab plus ipilimumab, 89% had one or more side effects compared with 57% of people treated with placebo. The most common side effects related to nivolumab plus ipilimumab treatment (occurring in ≥ 15% of people) were pruritus, fatigue, rash, diarrhea, hyperthyroidism, and hypothyroidism. Side effects related to treatment that were severe or very severe occurred in 28% of people treated with nivolumab plus ipilimumab and 2% of people treated with placebo. There were four deaths (1%) in the nivolumab plus ipilimumab-treated group and none in the placebo-treated group that were considered related to treatment.
7 What were the Main Conclusions?
Adjuvant therapy with nivolumab plus ipilimumab did not improve disease-free survival versus placebo in people with RCC who were at high risk of their cancer returning after partial or radical nephrectomy.
Reference
Motzer RJ, Russo P, Grünwald V, Tomita Y, Zurawski B, Parikh O, et al. Adjuvant nivolumab plus ipilimumab versus placebo for localised renal cell carcinoma after nephrectomy (CheckMate 914): a double-blind, randomised, phase 3 trial. Lancet. 2023;401(10379):821–32. https://doi.org/10.1016/S0140-6736(22)02574-0 (The original publication is available at: https://www.thelancet.com/journals/lancet/article/PIIS0140-6736(22)02574-0/fulltext).
Acknowledgements
Summary prepared by Christina McManus, PhD, at Envision Pharma Group Limited and funded by Bristol Myers Squibb. Patients treated at Memorial Sloan Kettering Cancer Center were supported in part by Memorial Sloan Kettering Cancer Center Support Grant (Core Grant, number P30 CA008748).
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Funding
This study was sponsored by Bristol Myers Squibb and Ono Pharmaceutical.
Conflict of interest
Please see the original publication [1] for full author disclosure information.
Ethics approval, availability of data and material, and coding availability
Not applicable.
Author contribution
All authors had the opportunity to review this summary publication.
Consent for publication
All authors had final responsibility for the decision to submit for publication.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution-NonCommercial 4.0 International License, which permits any non-commercial use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by-nc/4.0/.
About this article
Cite this article
Motzer, R.J., Russo, P., Grünwald, V. et al. Summary of Research: Adjuvant Nivolumab Plus Ipilimumab Versus Placebo for Localized Renal Cell Carcinoma After Nephrectomy (CheckMate 914): A Double-Blind, Randomized, Phase 3 Trial. Targ Oncol 18, 639–641 (2023). https://doi.org/10.1007/s11523-023-00987-1
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11523-023-00987-1