Abstract
Background
Oncogenic drivers in solid tumors include aberrant activation of mesenchymal epithelial transition factor (MET) and AXL.
Objective
This study investigated the safety and antitumor activity of glesatinib, a multitargeted receptor tyrosine kinase inhibitor that inhibits MET and AXL at clinically relevant doses, in combination with erlotinib or docetaxel.
Patients and Methods
The phase I portion of this open-label, multicenter study included two parallel arms in which ascending doses of oral glesatinib (starting dose 96 mg/m2) were administered with erlotinib or docetaxel (starting doses 100 mg once daily and 50 mg/m2, respectively) using a modified 3 + 3 design. Maximum tolerated dose (MTD) was based on dose-limiting toxicities (DLTs) during the first 21-day treatment cycle. Enrollment focused on patients with solid tumor types typically associated with MET aberration and/or AXL overexpression. The primary objective was to determine the safety profile of the treatment combinations. Antitumor activity and pharmacokinetics (PK) were also assessed.
Results
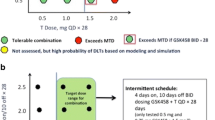
Ten dose levels of glesatinib across three glycolate formulations (unmicronized, micronized, or micronized version 2 [V2] tablets) available during the course of the study were investigated in 14 dose-escalation cohorts (n = 126). MTDs of unmicronized glesatinib plus erlotinib or docetaxel, and micronized glesatinib plus erlotinib were not reached. Micronized glesatinib 96 mg/m2 plus docetaxel exceeded the MTD. Further dosing focused on glesatinib micronized V2: maximum administered dose (MAD) was 700 mg twice daily with erlotinib 150 mg once daily or docetaxel 75 mg/m2 every 3 weeks. DLTs, acceptable at lower glesatinib (micronized V2) dose levels, occurred in two of five and two of six patients at the MADs of glesatinib + erlotinib and glesatinib + docetaxel, respectively. Across all cohorts, the most frequent treatment-related adverse events were diarrhea (glesatinib + erlotinib: 84.1%; glesatinib + docetaxel: 45.6%), fatigue (46.4%, 70.4%), and nausea (30.4%, 35.1%). The objective response rate was 1.8% and 12.0% in all glesatinib + erlotinib and glesatinib + docetaxel cohorts, respectively.
Conclusions
The safety profile of glesatinib plus erlotinib or docetaxel was acceptable and there were no PK interactions. MADs of glesatinib 700 mg twice daily (micronized V2) with erlotinib 150 mg once daily or docetaxel 75 mg/m2 every 3 weeks exceeded the MTD by a small margin. Modest signals of efficacy were observed with these treatment combinations in non-genetically selected patients with advanced solid tumors.
Clinical Trials Registration
ClinicalTrials.gov NCT00975767; 11 September 2009.


Similar content being viewed by others
Change history
05 April 2022
A Correction to this paper has been published: https://doi.org/10.1007/s11523-022-00877-y
References
Robinson KW, Sandler AB. The role of MET receptor tyrosine kinase in non-small cell lung cancer and clinical development of targeted anti-MET agents. Oncologist. 2013;18(2):115–22. https://doi.org/10.1634/theoncologist.2012-0262.
Rehman S, Dy GK. MET inhibition in non-small cell lung cancer. Eur Med J. 2018;4(1):100–11.
Jeon HM, Lee J. MET: roles in epithelial-mesenchymal transition and cancer stemness. Ann Transl Med. 2017;5(1):5. https://doi.org/10.21037/atm.2016.12.67.
Ariyawutyakorn W, Saichaemchan S, Varella-Garcia M. Understanding and targeting MET signaling in solid tumors: are we there yet? J Cancer. 2016;7(6):633–49. https://doi.org/10.7150/jca.12663.
Cancer Genome Atlas Research Network. Comprehensive molecular profiling of lung adenocarcinoma. Nature. 2014;511(7511):543–50. https://doi.org/10.1038/nature13385.
Tong JH, Yeung SF, Chan AW, Chung LY, Chau SL, Lung RW, et al. MET amplification and exon 14 splice site mutation define unique molecular subgroups of non-small cell lung carcinoma with poor prognosis. Clin Cancer Res. 2016;22(12):3048–56. https://doi.org/10.1158/1078-0432.CCR-15-2061.
Moosavi F, Giovannetti E, Saso L, Firuzi O. HGF/MET pathway aberrations as diagnostic, prognostic, and predictive biomarkers in human cancers. Crit Rev Clin Lab Sci. 2019;56(8):533–66. https://doi.org/10.1080/10408363.2019.1653821.
Wang Q, Yang S, Wang K, Sun SY. MET inhibitors for targeted therapy of EGFR TKI-resistant lung cancer. J Hematol Oncol. 2019;12(1):63. https://doi.org/10.1186/s13045-019-0759-9.
Linklater ES, Tovar EA, Essenburg CJ, Turner L, Madaj Z, Winn ME, et al. Targeting MET and EGFR crosstalk signaling in triple-negative breast cancers. Oncotarget. 2016;7(43):69903–15. https://doi.org/10.18632/oncotarget.12065.
Sequist LV, Han JY, Ahn MJ, Cho BC, Yu H, Kim SW, et al. Osimertinib plus savolitinib in patients with EGFR mutation-positive, MET-amplified, non-small-cell lung cancer after progression on EGFR tyrosine kinase inhibitors: interim results from a multicentre, open-label, phase 1b study. Lancet Oncol. 2020;21(3):373–86. https://doi.org/10.1016/S1470-2045(19)30785-5.
Morgillo F, Della Corte CM, Fasano M, Ciardiello F. Mechanisms of resistance to EGFR-targeted drugs: lung cancer. ESMO Open. 2016;1(3): e000060. https://doi.org/10.1136/esmoopen-2016-000060.
Zhang G, Wang M, Zhao H, Cui W. Function of Axl receptor tyrosine kinase in non-small cell lung cancer. Oncol Lett. 2018;15(3):2726–34. https://doi.org/10.3892/ol.2017.7694.
Sato K, Suda K, Shimizu S, Sakai K, Mizuuchi H, Tomizawa K, et al. Clinical, pathological, and molecular features of lung adenocarcinomas with AXL expression. PLoS One. 2016;11(4): e0154186. https://doi.org/10.1371/journal.pone.0154186.
Ishikawa M, Sonobe M, Nakayama E, Kobayashi M, Kikuchi R, Kitamura J, et al. Higher expression of receptor tyrosine kinase Axl, and differential expression of its ligand, Gas6, predict poor survival in lung adenocarcinoma patients. Ann Surg Oncol. 2013;20(Suppl 3):S467–76. https://doi.org/10.1245/s10434-012-2795-3.
Hsieh MS, Yang PW, Wong LF, Lee JM. The AXL receptor tyrosine kinase is associated with adverse prognosis and distant metastasis in esophageal squamous cell carcinoma. Oncotarget. 2016;7(24):36956–70. https://doi.org/10.18632/oncotarget.9231.
Lozneanu L, Pinciroli P, Ciobanu DA, Carcangiu ML, Canevari S, Tomassetti A, et al. Computational and immunohistochemical analyses highlight AXL as a potential prognostic marker for ovarian cancer patients. Anticancer Res. 2016;36(8):4155–63.
Flem-Karlsen K, Nyakas M, Farstad IN, McFadden E, Wernhoff P, Jacobsen KD, et al. Soluble AXL as a marker of disease progression and survival in melanoma. PLoS One. 2020;15(1): e0227187. https://doi.org/10.1371/journal.pone.0227187.
Tanaka K, Tokunaga E, Inoue Y, Yamashita N, Saeki H, Okano S, et al. Impact of expression of vimentin and Axl in breast cancer. Clin Breast Cancer. 2016;16(6):520-6.e2. https://doi.org/10.1016/j.clbc.2016.06.015.
Taniguchi H, Yamada T, Wang R, Tanimura K, Adachi Y, Nishiyama A, et al. AXL confers intrinsic resistance to osimertinib and advances the emergence of tolerant cells. Nat Commun. 2019;10(1):259. https://doi.org/10.1038/s41467-018-08074-0.
Engstrom LD, Aranda R, Lee M, Tovar EA, Essenburg CJ, Madaj Z, et al. Glesatinib exhibits antitumor activity in lung cancer models and patients harboring MET exon 14 mutations and overcomes mutation-mediated resistance to type I MET inhibitors in nonclinical models. Clin Cancer Res. 2017;23(21):6661–72. https://doi.org/10.1158/1078-0432.CCR-17-1192.
Eisenhauer EA, Therasse P, Bogaerts J, Schwartz LH, Sargent D, Ford R, et al. New response evaluation criteria in solid tumours: revised RECIST guideline (version 1.1). Eur J Cancer. 2009;45(2):228–47. https://doi.org/10.1016/j.ejca.2008.10.026.
Savjani KT, Gajjar AK, Savjani JK. Drug solubility: importance and enhancement techniques. ISRN Pharm. 2012;2012: 195727. https://doi.org/10.5402/2012/195727.
Brake K, Gumireddy A, Tiwari A, Chauhan H. In vivo studies for drug development via oral delivery: challenges, animal models and techniques. Pharm Anal Acta. 2017;8:1000560. https://doi.org/10.4172/2153-2435.1000560.
Acknowledgments
The authors thank the patients and their families who participated in this study. They also thank Josée Morin (Excelsus Statistics Inc.) for critical review of the manuscript draft and Michel Drouin (formally of Methylgene Inc.) for contribution to the study design. Medical writing services were provided by Siân Marshall (SIANTIFIX, Cambridge, UK) in accordance with Good Publication Practice (GPP3) guidelines (http://www.ismpp.org/gpp3) and funded by Mirati Therapeutics, Inc.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Author contributions
Study conception and design: MT, RC, VT. Data acquisition: AP, SG, KPP, DWR, NBH, PJO. Data analysis and interpretation: All authors. Manuscript reviewing and editing: All authors. Manuscript original draft: No authors, see medical writing acknowledgment. All authors reviewed and approved the final draft of the manuscript for publication and agree to be accountable for all aspects of this work.
Funding
This study (NCT00975767) was supported by Mirati Therapeutics Inc. Funding for a professional medical writer with access to the data was provided by Mirati Therapeutics Inc.
Conflict of interest
Amita Patnaik reports honoraria from the Texas Society of Clinical Oncology; consulting fees (personal) from Bayer, Daiichi Sankyo, Gilead Sciences, HalioDx, Merck, Novartis, Seattle Genetics, Shenzhen IONOVA Life Sciences, and Silverback Therapeutics; consulting fees (to an immediate family member) from Bristol Meyers Squibb, Genentech/Roche, and Merck; and research funding from Abbvie, Arcus Ventures, Astellas Pharma, Bolt Biotherapeutics, Corvus Pharmaceuticals, Daiichi-Sankyo, Exelixis, Fochon Pharmaceuticals, Five Prime Therapeutics, FortySeven, Gilead Sciences, Infinity Pharmaceuticals, Inova, Klus Pharma, Lilly, Livzon, Merck, Pfizer, Pieris Pharmaceuticals, Plexxikon, Sanofi, Seattle Genetics, Surface Oncology, Symphogen, Syndax, Tesaro, Upsher-Smith, and Viego Therapeutics. Shirish Gadgeel reports consulting fees or honorarium from AstraZeneca, Blueprint Medicines, Bristol Meyers Squibb, Eli Lilly, Genentech-Roche, Janssen, Mirati Therapeutics, Novartis, and Pfizer; support for travel to meetings for study manuscript preparation from Genetech-Roche and Merck; fees for participating in review activities from AstraZeneca; and provision of writing assistance from Genentech-Roche and Pfizer. Kyriakos Papadopoulos reports consulting fees from Bicycle Therapeutics and Turning Point Therapeutics; fees for review activities from Basilea Pharmaceutica; and funding for clinical trial conduct from Abbvie, ADC Therapeutics, Amgen, Bayer, Clithera Biosciences, Daiichi Sankyo, EMD Serono, F-star Therapeutics, Incyte, Jounce Therapeutics, Linnaeus Therapeutics, Loxo Oncology, MabSpace Biosciences, 3D Medicines, MedImmune, Merck, Mersana Therapeutics, Mirati Therapeutics, Pfizer, Peloton Therapeutics, Regeneron Pharmaceuticals, Syros Pharmaceuticals, Tempest Therapeutics, and Treadwell. Drew Rasco reports research funding from Mirati Therapeutics. Naomi Haas has no potential conflicts of interest to disclose. Peter O’Dwyer reports research support from Array Biopharma, AstraZeneca, BBI, Bristol Meyers Squibb, Celgene, Five Prime Therapeutics, FortySeven, Genentech, GSK, H3 Biomedicine, Incyte, Lilly (Imclone), Merck, Minneamrita Therapeutics, Mirati Therapeutics, Novartis, Pfizer, Pharmacyclics (AbbVie), Syndax Pharmaceuticals, and Taiho Pharma; consulting fees from Array Biopharma and Genentech; and expert testimonial fees from Bayer and Lilly. Richard Chao, Hirak Der-Torossian, and Vanessa Tassell report employment and stock ownership for Mirati Therapeutics. Diane Potvin reports employment for Mirati Therapeutics. Demiana Faltaos reports prior employment and stock ownership for Mirati Therapeutics. Manal Tawashi reports prior employment for Mirati Therapeutics.
Data availability
Requests for data underlying the findings described in this article are available following reasonable request to the corresponding author.
Code availability
Not applicable.
Ethics approval
This study was conducted in accordance with the Declaration of Helsinki, International Conference on Harmonisation Guidelines for Good Clinical Practice, and local regulatory requirements. The study protocol was approved by the Institutional Review Boards at each participating study site.
Consent to participate
Patients provided written, informed consent
Consent for publication
All authors gave final approval of the version to be published.
Additional information
The original article has been updated: Due to title change.
Rights and permissions
About this article
Cite this article
Patnaik, A., Gadgeel, S., Papadopoulos, K.P. et al. Phase I Study of Glesatinib (MGCD265) in Combination with Erlotinib or Docetaxel in Patients with Advanced Solid Tumors. Targ Oncol 17, 125–138 (2022). https://doi.org/10.1007/s11523-022-00875-0
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11523-022-00875-0