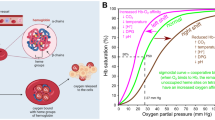
Abstract
The blood plays a vital role in the human body and serves as an intermediary between various physiological systems and organs. White blood cells, which are a part of the immune system, defend against infections and regulate the body temperature and pH balance. Blood platelets play a crucial role in clotting, the prevention of excessive bleeding, and the promotion of healing. Blood also serves as a courier system that transports hormones to facilitate communication and synchronization between different organs and systems in the body. The circulatory system, comprised of arteries, veins, and capillaries, plays a crucial role in the efficient transportation and connection of vital nutrients and oxygen. Despite the importance of natural blood, there are often supply shortages, compatibility issues, and medical conditions, which make alternatives such as artificial blood necessary. This is particularly relevant in cancer treatment, which was the focus of our study. In this study, we investigated the potential of artificial blood in cancer therapy, specifically to address tumor hypoxia. We also examined the potential of red blood cell substitutes such as hemoglobin-based oxygen carriers and perfluorocarbons. Additionally, we examined the production of hemoglobin using E. coli and the role of hemoglobin in oncogenesis. Furthermore, we explored the potential use of artificial platelets for cancer treatment. Our study emphasizes the significance of artificial blood in improving cancer treatment outcomes.
Graphical Abstract





Similar content being viewed by others
Data availability
All the data pertaining to this study have been provided in the manuscript.
Abbreviations
- AOC:
-
Artificial oxygen carrier
- CBZ:
-
Cabazitaxel
- CSA:
-
Canine serum albumin
- Hb:
-
Hemoglobin
- HBOCs:
-
Hemoglobin-based oxygen carriers
- HCC:
-
Hepatocellular carcinoma
- HIF:
-
Hypoxia-inducible factor
- LEH:
-
Liposome-encapsulated hemoglobin
- MRI:
-
Magnetic resonance imaging
- PBOC/PFOC:
-
Perfluorocarbon-based oxygen carriers
- PEG:
-
Polyethylene glycol
- PEG-Hb:
-
PEG-conjugated hemoglobin
- PFC:
-
Perfluorocarbons
- PolyHb:
-
Polymeric hemoglobin
- PolyHbBv:
-
Polymerized bovine hemoglobin
- RBCs:
-
Red blood cells
- RBS:
-
Red blood substitutes
- rCSA:
-
Recombinant canine serum albumin
- RT:
-
Radiotherapy
- VEGFR:
-
Vascular endothelial growth factor
- WBC:
-
White blood cells
References
Kresie L (2001) Artificial blood: an update on current red cell and platelet substitutes. Proc (Baylor Univ Med Cent) 14(2):158
Sarkar S (2008) Artificial blood. Indian J Crit Care Med 12(3):140–144
Paul M (2021) A review on artificial blood. J Blood Disord Transfus 12:481
Khan F, Singh K, Friedman MT (2020) Artificial blood: the history and current perspectives of blood substitutes. Discoveries 8(1):e104
Krishnia L, Kashyap MK (2024) Development of compendium for esophageal squamous cell carcinoma. J Vis Exp (206):e65480
Sharma R, Sharma S (2023) Physiology, Blood Volume. In: StatPearls [Internet]. Treasure Island (FL): StatPearls Publishing; 2024 Jan-. Available from: https://www.ncbi.nlm.nih.gov/books/NBK526077/
Dean L (2005) Blood groups and red cell antigens: NCBI
Haldar R, Gupta D, Chitranshi S, Singh MK, Sachan S (2019) Artificial blood: a futuristic dimension of modern day transfusion sciences. Cardiovasc Hematol Agents Med Chem 17(1):11–16
Yamada K, Yokomaku K, Kureishi M, Akiyama M, Kihira K, Komatsu T (2016) Artificial blood for dogs. Sci Rep 6:36782
Choi MY, Kashyap MK, Kumar D (2016) The chronic lymphocytic leukemia microenvironment: beyond the B-cell receptor. Best Pract Res Clin Haematol 29(1):40–53
Kashyap MK, Amaya-Chanaga CI, Kumar D, Simmons B, Huser N, Gu Y et al (2017) Targeting the CXCR4 pathway using a novel anti-CXCR4 IgG1 antibody (PF-06747143) in chronic lymphocytic leukemia. J Hematol Oncol 10(1):112
Al Tameemi W, Dale TP, Al-Jumaily RMK, Forsyth NR (2019) Hypoxia-modified cancer cell metabolism. Front Cell Dev Biol 7:4
Paredes F, Williams HC, San MA (2021) Metabolic adaptation in hypoxia and cancer. Cancer Lett 502:133–142
Ziello JE, Jovin IS, Huang Y (2007) Hypoxia-inducible factor (HIF)-1 regulatory pathway and its potential for therapeutic intervention in malignancy and ischemia. Yale J Biol Med 80(2):51–60
Liu ZL, Chen HH, Zheng LL, Sun LP, Shi L (2023) Angiogenic signaling pathways and anti-angiogenic therapy for cancer. Signal Transduct Target Ther 8(1):198
Chen Z, Han F, Du Y, Shi H, Zhou W (2023) Hypoxic microenvironment in cancer: molecular mechanisms and therapeutic interventions. Signal Transduct Target Ther 8(1):70
Li Y, Zhao L, Li XF (2021) Hypoxia and the tumor microenvironment. Technol Cancer Res Treat 20:15330338211036304
Emami Nejad A, Najafgholian S, Rostami A, Sistani A, Shojaeifar S, Esparvarinha M et al (2021) The role of hypoxia in the tumor microenvironment and development of cancer stem cell: a novel approach to developing treatment. Cancer Cell Int 21(1):62
Alimoradi H, Matikonda SS, Gamble AB, Giles GI, Greish K (2016) Hypoxia responsive drug delivery systems in tumor therapy. Curr Pharm Des 22(19):2808–2820
Shi R, Liao C, Zhang Q (2021) Hypoxia-driven effects in cancer: characterization, mechanisms, and therapeutic implications. Cells 10(3):678
Vaupel P, Harrison L (2004) Tumor hypoxia: causative factors, compensatory mechanisms, and cellular response. Oncologist 9(Suppl 5):4–9
Jun JC, Rathore A, Younas H, Gilkes D, Polotsky VY (2017) Hypoxia-inducible factors and cancer. Curr Sleep Med Rep 3(1):1–10
Teicher BA (1994) Hypoxia and drug resistance. Cancer Metastasis Rev 13(2):139–168
Gao M, Liang C, Song X, Chen Q, Jin Q, Wang C et al (2017) Erythrocyte-membrane-enveloped perfluorocarbon as nanoscale artificial red blood cells to relieve tumor hypoxia and enhance cancer radiotherapy. Adv Mater 29(35):1701429
Alayash AI (2014) Blood substitutes: why haven’t we been more successful? Trends Biotechnol 32(4):177–185
Wu W, Yang Q, Wu W, Yang Q, Li T, Zhang P et al (2009) Hemoglobin-based oxygen carriers combined with anticancer drugs may enhance sensitivity of radiotherapy and chemotherapy to solid tumors. Artif Cells Blood Substitut Biotechnol 37(4):163–165
Gottschalk A, Raabe A, Hommel M, Rempf C, Freitag M, Standl T (2005) Influence of the hemoglobin solution HBOC-201 on tissue oxygenation in the rat R1H-tumor. Artif Cells Blood Substit Immobil Biotechnol 33(4):379–389
Qi X, Wong BL, Lau SH, Ng KT, Kwok SY, Kin-Wai Sun C et al (2017) A hemoglobin-based oxygen carrier sensitized Cisplatin based chemotherapy in hepatocellular carcinoma. Oncotarget 8(49):85311–85325
Chow E, Lau JSH, Wai T, Lam IPY (2021) The anti-tumoral effects of the oxygen carrier YQ23 in a triple-negative breast cancer syngeneic model. Transl Cancer Res 10(2):656–668
Lucas A, Belcher DA, Munoz C, Williams AT, Palmer AF, Cabrales P (2020) Polymerized human hemoglobin increases the effectiveness of cisplatin-based chemotherapy in non-small cell lung cancer. Oncotarget 11(42):3770–3781
Li B, Zhang J, Ma N, Li W, You G, Chen G et al (2023) PEG-conjugated bovine haemoglobin enhances efficiency of chemotherapeutic agent doxorubicin with alleviating DOX-induced splenocardiac toxicity in the breast cancer. Artif Cells Nanomed Biotechnol 51(1):120–130
Dai M, Yu M, Han J, Li H, Cui P, Liu Q et al (2008) PEG-conjugated hemoglobin combination with cisplatin enforced the antiangiogeic effect in a cervical tumor xenograft model. Artif Cells Blood Substit Immobil Biotechnol 36(6):487–497
Han J, Yu M, Dai M, Cui P, Li H, Zhang J et al (2008) Effect of artificial oxygen carrier with chemotherapy on tumor hypoxia and neovascularization. Artif Cells Blood Substit Immobil Biotechnol 36(5):431–438
Chang TM (1964) Semipermeable Microcapsules. Science 146(3643):524–525
Chang TMS. Artificial cells: Charles C. Thomas Publisher; 1972.
Chang TM (1971) Stabilisation of enzymes by microencapsulation with a concentrated protein solution or by microencapsulation followed by cross-linking with glutaraldehyde. Biochem Biophys Res Commun 44(6):1531–1536
Chang TMS (2007) Monograph on artificial cells: biotechnology, nanotechnology. Regenerative Medicine, Bioencapsulation, Cell/Stem Cell Therapy World Science Publisher, Blood Substitutes
Chang TMS. Blood substitutes: principles, methods, products and clinical trials: Vol. 2: S.Karger AG; 1998.
Gould SA, Moore EE, Hoyt DB, Ness PM, Norris EJ, Carson JL, et al. The life-sustaining capacity of human polymerized hemoglobin when red cells might be unavailable. Journal of the American College of Surgeons. 2002;195(4):445–52; discussion 52–5.
Pearce LB, Gawryl MS, Rentko VT, Moon-Massat PF, Rausch CW. HBOC-201 (hemoglobin glutamer-250)(bovine), hemopure®): clinical studies. Blood substitutes. 2006:437–50.
Jiang MS, Yin XY, Qin B, Xuan SY, Yuan XL, Yin H et al (2019) Inhibiting hypoxia and chemotherapy-induced cancer cell metastasis under a valid therapeutic effect by an assistance of biomimetic oxygen delivery. Mol Pharm 16(11):4530–4541
Xia Q, Zhang Y, Li Z, Hou X, Feng N (2019) Red blood cell membrane-camouflaged nanoparticles: a novel drug delivery system for antitumor application. Acta pharmaceutica Sinica B 9(4):675–689
Murayama C, Kawaguchi AT, Ishikawa K, Kamijo A, Kato N, Ohizumi Y et al (2012) Liposome-encapsulated hemoglobin ameliorates tumor hypoxia and enhances radiation therapy to suppress tumor growth in mice. Artif Organs 36(2):170–177
Jain RK (1990) Physiological barriers to delivery of monoclonal antibodies and other macromolecules in tumors. Cancer Res 50:814s–9s
Natarajan C, Signore AV, Kumar V, Storz JF (2020) Synthesis of recombinant human hemoglobin with NH(2) -terminal acetylation in Escherichia coli. Curr Protoc Protein Sci 101(1):e112
Natarajan C, Jiang X, Fago A, Weber RE, Moriyama H, Storz JF (2011) Expression and purification of recombinant hemoglobin in Escherichia coli. PLoS ONE 6(5):e20176
Jagers J, Wrobeln A, Ferenz KB (2021) Perfluorocarbon-based oxygen carriers: from physics to physiology. Pflugers Arch 473(2):139–150
Krafft MP (2020) Alleviating tumor hypoxia with perfluorocarbon-based oxygen carriers. Curr Opin Pharmacol 53:117–125
Feldman LA, Fabre MS, Grasso C, Reid D, Broaddus WC, Lanza GM et al (2017) Perfluorocarbon emulsions radiosensitise brain tumors in carbogen breathing mice with orthotopic GL261 gliomas. PLoS ONE 12(9):e0184250
Guo R, Xu N, Liu Y, Ling G, Yu J, Zhang P (2021) Functional ultrasound-triggered phase-shift perfluorocarbon nanodroplets for cancer therapy. Ultrasound Med Biol 47(8):2064–2079
Lowe KC (1999) Perfluorinated blood substitutes and artificial oxygen carriers. Blood Rev 13(3):171–184
Donahue LL, Shapira I, Shander A, Kolitz J, Allen S, Greenburg G (2010) Management of acute anemia in a Jehovah’s Witness patient with acute lymphoblastic leukemia with polymerized bovine hemoglobin-based oxygen carrier: a case report and review of literature. Transfusion 50(7):1561–1567
Peila R, Rohan TE (2020) Diabetes, glycated hemoglobin, and risk of cancer in the UK Biobank Study. Cancer Epidemiol Biomarkers Prev 29(6):1107–1119
Chi G, Lee JJ, Montazerin SM, Marszalek J (2022) Prognostic value of hemoglobin-to-red cell distribution width ratio in cancer: a systematic review and meta-analysis. Biomark Med 16(6):473–482
Kang N, Qiu WJ, Wang B, Tang DF, Shen XY (2022) Role of hemoglobin alpha and hemoglobin beta in non-small-cell lung cancer based on bioinformatics analysis. Mol Carcinog 61(6):587–602
Blair S, Barlow C, Martin E, Schumaker R, McIntyre J (2020) Methemoglobin determination by multi-component analysis in coho salmon (Oncorhynchus kisutch) possessing unstable hemoglobin. MethodsX 7:100836
Carrola A, Romao CC, Vieira HLA (2023) Carboxyhemoglobin (COHb): unavoidable bystander or protective player? Antioxidants 12(6):1198
Kuleshova ID, Zaripov PI, Poluektov YM, Anashkina AA, Kaluzhny DN, Parshina EY et al (2023) Changes in Hemoglobin properties in complex with glutathione and after glutathionylation. Int J Mol Sci 24(17):13557
Cortazzo JA, Lichtman AD (2014) Methemoglobinemia: a review and recommendations for management. J Cardiothorac Vasc Anesth 28(4):1043–1047
Frimat M, Boudhabhay I, Roumenina LT (2019) Hemolysis derived products toxicity and endothelium: model of the second hit. Toxins 11(11):660
Muz B, de la Puente P, Azab F, Azab AK (2015) The role of hypoxia in cancer progression, angiogenesis, metastasis, and resistance to therapy. Hypoxia 3:83–92
Qiu GZ, Jin MZ, Dai JX, Sun W, Feng JH, Jin WL (2017) Reprogramming of the tumor in the hypoxic niche: the emerging concept and associated therapeutic strategies. Trends Pharmacol Sci 38(8):669–686
Wilson WR, Hay MP (2011) Targeting hypoxia in cancer therapy. Nat Rev Cancer 11(6):393–410
Jiang M, Yu CH, Xu Z, Qin Z (2024) Binding of carbon monoxide to hemoglobin in an oxygen environment: force field development for molecular dynamics. J Chem Theory Comput 20(10):4229–4238
Wegiel B, Gallo D, Csizmadia E, Harris C, Belcher J, Vercellotti GM et al (2013) Carbon monoxide expedites metabolic exhaustion to inhibit tumor growth. Cancer Res 73(23):7009–7021
Nakamura H, Takada K (2021) Reactive oxygen species in cancer: current findings and future directions. Cancer Sci 112(10):3945–3952
Luu Hoang KN, Anstee JE, Arnold JN (2021) The diverse roles of heme oxygenase-1 in tumor progression. Front Immunol 12:658315
Semenza GL (2012) Hypoxia-inducible factors in physiology and medicine. Cell 148(3):399–408
Harris AL (2002) Hypoxia–a key regulatory factor in tumour growth. Nat Rev Cancer 2(1):38–47
Hassan Venkatesh G, Abou Khouzam R, Shaaban Moustafa Elsayed W, Ahmed Zeinelabdin N, Terry S, Chouaib S (2021) Tumor hypoxia: an important regulator of tumor progression or a potential modulator of tumor immunogenicity? Oncoimmunology. 10:1974233
Teicher BA, Herman TS, Hopkins RE, Menon K (1991) Effect of oxygen level on the enhancement of tumor response to radiation by perfluorochemical emulsions or a bovine hemoglobin preparation. Int J Radiat Oncol Biol Phys 21(4):969–974
Teicher BA, Herman TS, Menon K (1992) Enhancement of fractionated radiation therapy by an experimental concentrated perflubron emulsion (Oxygent) in the Lewis lung carcinoma. Biomater Artif Cells Immobilization Biotechnol 20(2–4):899–902
Teicher BA, Holden SA, Dupuis NP, Kusomoto T, Liu M, Liu F et al (1994) Oxygenation of the rat 9L gliosarcoma and the rat 13672 mammary carcinoma with various doses of a hemoglobin solution. Artif Cells Blood Substit Immobil Biotechnol 22(3):827–833
Nozue M, Lee I, Manning JM, Manning LR, Jain RK (1996) Oxygenation in tumors by modified hemoglobins. J Surg Oncol 62(2):109–114
Raabe A, Gottschalk A, Hommel M, Dubben HH, Strandl T (2005) No effect of the hemoglobin solution HBOC-201 on the response of the rat R1H tumor to fractionated irradiation. Strahlentherapie und Onkologie : Organ der Deutschen Rontgengesellschaft [et al] 181(11):730–737
Yu M, Han J, Dai M, Cui P, Li H, Liu Q et al (2008) Influence of PEG-conjugated hemoglobin on tumor oxygenation and response to chemotherapy. Artif Cells Blood Substit Immobil Biotechnol 36(6):551–561
Yamamoto M, Izumi Y, Horinouchi H, Teramura Y, Sakai H, Kohno M et al (2009) Systemic administration of hemoglobin vesicle elevates tumor tissue oxygen tension and modifies tumor response to irradiation. J Surg Res 151(1):48–54
Han J, Yu M, Dai M, Li H, Xiu R, Liu Q (2012) Decreased expression of MDR1 in PEG-conjugated hemoglobin solution combined cisplatin treatment in a tumor xenograft model. Artif Cells Blood Substit Immobil Biotechnol 40(4):239–244
Liu XB, Cheng Q, Geng W, Ling CC, Liu Y, Ng KT et al (2014) Enhancement of cisplatin-based TACE by a hemoglobin-based oxygen carrier in an orthotopic rat HCC model. Artif Cells Nanomed Biotechnol 42(4):229–236
Li CX, Wong BL, Ling CC, Ma YY, Shao Y, Geng W et al (2014) A novel oxygen carrier “YQ23” suppresses the liver tumor metastasis by decreasing circulating endothelial progenitor cells and regulatory T cells. BMC Cancer 14:293
Lee NP, Chan KT, Choi MY, Lam HY, Tung LN, Tzang FC et al (2015) Oxygen carrier YQ23 can enhance the chemotherapeutic drug responses of chemoresistant esophageal tumor xenografts. Cancer Chemother Pharmacol 76(6):1199–1207
Kawaguchi F, Kawaguchi AT, Murayama C, Kamijo A, Haida M (2017) Liposome-encapsulated hemoglobin improves tumor oxygenation as detected by near-infrared spectroscopy in colon carcinoma in Mice. Artif Organs 41(4):327–335
Belcher DA, Ju JA, Baek JH, Yalamanoglu A, Buehler PW, Gilkes DM et al (2018) The quaternary state of polymerized human hemoglobin regulates oxygenation of breast cancer solid tumors: a theoretical and experimental study. PLoS ONE 13(2):e0191275
Zhao Y, Chen G, Meng Z, Gong G, Zhao W, Wang K et al (2019) A novel nanoparticle drug delivery system based on PEGylated hemoglobin for cancer therapy. Drug Delivery 26(1):717–723
Teicher BA, Herman TS, Jones SM (1989) Optimization of perfluorochemical levels with radiation therapy in mice. Cancer Res 49(10):2693–2697
Silkstone GG, Silkstone RS, Wilson MT, Simons M, Bulow L, Kallberg K et al (2016) Engineering tyrosine electron transfer pathways decreases oxidative toxicity in hemoglobin: implications for blood substitute design. Biochem J 473(19):3371–3383
Gutteridge JM, Halliwell B (2000) Free radicals and antioxidants in the year 2000. A historical look to the future. Ann N Y Acad Sci 899:136–47
Sakai H, Kure T, Taguchi K, Azuma H (2022) Research of storable and ready-to-use artificial red blood cells (hemoglobin vesicles) for emergency medicine and other clinical applications. Frontiers in medical technology 4:1048951
Sen Gupta A (2017) Bio-inspired nanomedicine strategies for artificial blood components. Wiley Interdiscip Rev Nanomed Nanobiotechnol Wiley Interdiscip Rev Nanomed Nanobiotechnol 9(6):10.1002/wnan.1464
Gupta V, Bhavanasi S, Quadir M, Singh K, Ghosh G, Vasamreddy K et al (2019) Protein PEGylation for cancer therapy: bench to bedside. J Cell Commun Signal 13(3):319–330
Li X, Peng X, Zoulikha M, Boafo GF, Magar KT, Ju Y et al (2024) Multifunctional nanoparticle-mediated combining therapy for human diseases. Signal Transduct Target Ther 9(1):1
Samuel Tillmans SW. Synthetic and recombinant hemoglobins. Basic Anesthesia Review Oxford University Press; 2024. p. 753.
Mohanto N, Park YJ, Jee JP (2023) Current perspectives of artificial oxygen carriers as red blood cell substitutes: a review of old to cutting-edge technologies using in vitro and in vivo assessments. J Pharm Investig 53(1):153–190
Lyons TJ, Basu A (2012) Biomarkers in diabetes: hemoglobin A1c, vascular and tissue markers. Transl Res 159(4):303–312
Zheng J, Gao Y, Xie SH, Santoni G, Lagergren J (2022) Haemoglobin A1c and serum glucose levels and risk of gastric cancer: a systematic review and meta-analysis. Br J Cancer 126(7):1100–1107
Hope C, Robertshaw A, Cheung KL, Idris I, English E (2016) Relationship between HbA1c and cancer in people with or without diabetes: a systematic review. Diabet Med 33(8):1013–1025
de Beer JC, Liebenberg L (2014) Does cancer risk increase with HbA1c, independent of diabetes? Br J Cancer 110(9):2361–2368
Venturini E, Iannuzzo G, D'Andrea A, Pacileo M, Tarantini L, Canale ML et al (2020) Oncology and cardiac rehabilitation: an underrated relationship. J Clin Med 9(6):1810
Byun YH, Kim SY, Mok Y, Kim Y, Jee SH (2018) Heart rate recovery and cancer risk: prospective cohort study. Asia Pac J Public Health 30(1):45–55
Busti F, Marchi G, Ugolini S, Castagna A, Girelli D (2018) Anemia and iron deficiency in cancer patients: role of iron replacement therapy. Pharmaceuticals 11(4):94
Groopman JE, Itri LM (1999) Chemotherapy-induced anemia in adults: incidence and treatment. J Natl Cancer Inst 91(19):1616–1634
Madeddu C, Gramignano G, Astara G, Demontis R, Sanna E, Atzeni V et al (2018) Pathogenesis and treatment options of cancer related anemia: perspective for a targeted mechanism-based approach. Front Physiol 9:1294
Podkalicka P, Mucha O, Jozkowicz A, Dulak J, Loboda A (2018) Heme oxygenase inhibition in cancers: possible tools and targets. Contemp Oncol 22(1A):23–32
Weidemann A, Johnson RS (2008) Biology of HIF-1alpha. Cell Death Differ 15(4):621–627
Kurota Y, Takeda Y, Ichiyanagi O, Saitoh S, Ito H, Naito S et al (2023) Hemoglobin beta expression is associated with poor prognosis in clear cell renal cell carcinoma. Biomedicines 11(5):1330
Borsi E, Perrone G, Terragna C, Martello M, Dico AF, Solaini G et al (2014) Hypoxia inducible factor-1 alpha as a therapeutic target in multiple myeloma. Oncotarget 5(7):1779–1792
Papizan JB, Porter SN, Sharma A, Pruett-Miller SM (2020) Therapeutic gene editing strategies using CRISPR-Cas9 for the beta-hemoglobinopathies. J Biomed Res 35(2):115–134
Esrick EB, Lehmann LE, Biffi A, Achebe M, Brendel C, Ciuculescu MF et al (2021) Post-transcriptional genetic silencing of BCL11A to treat sickle cell disease. N Engl J Med 384(3):205–215
Zheng Y, Miyamoto DT, Wittner BS, Sullivan JP, Aceto N, Jordan NV et al (2017) Expression of beta-globin by cancer cells promotes cell survival during blood-borne dissemination. Nat Commun 8:14344
Luc NF, Rohner N, Girish A, Didar Singh Sekhon U, Neal MD, Sen Gupta A (2022) Bioinspired artificial platelets: past, present and future. Platelets. 33(1):35–47
Rybak ME, Renzulli LA (1993) A liposome based platelet substitute, the plateletsome, with hemostatic efficacy. Biomater Artif Cells Immobilization Biotechnol 21(2):101–118
Graham SS, Gonchoroff NJ, Miller JL (2001) Infusible platelet membranes retain partial functionality of the platelet GPIb/IX/V receptor complex. Am J Clin Pathol 115(1):144–147
Nasiri S (2013) Infusible platelet membrane as a platelet substitute for transfusion: an overview. Blood Transfus 11:337–42
Fitzpatrick GM, Cliff R, Tandon N (2013) Thrombosomes: a platelet-derived hemostatic agent for control of noncompressible hemorrhage. Transfusion 53(Suppl 1):100S-S106
Bynum JA, Meledeo MA, Peltier GC, McIntosh CS, Taylor AS, Montgomery RK et al (2019) Evaluation of a lyophilized platelet-derived hemostatic product. Transfusion 59(S2):1490–1498
Agam G, Livne A (1983) Passive participation of fixed platelets in aggregation facilitated by covalently bound fibrinogen. Blood 61(1):186–191
Sung AD, Yen RC, Jiao Y, Bernanke A, Lewis DA, Miller SE et al (2020) Fibrinogen-coated albumin nanospheres prevent thrombocytopenia-related bleeding. Radiat Res 194(2):162–172
Verhoef C, Singla N, Moneta G, Muir W, Rijken A, Lockstadt H et al (2015) Fibrocaps for surgical hemostasis: two randomized, controlled phase II trials. J Surg Res 194(2):679–687
Doshi N, Orje JN, Molins B, Smith JW, Mitragotri S, Ruggeri ZM (2012) Platelet mimetic particles for targeting thrombi in flowing blood. Adv Mater 24(28):3864–3869
Takeoka S, Teramura Y, Okamura Y, Tsuchida E, Handa M, Ikeda Y (2002) Rolling properties of rGPIbalpha-conjugated phospholipid vesicles with different membrane flexibilities on vWf surface under flow conditions. Biochem Biophys Res Commun 296(3):765–770
Nishiya T, Kainoh M, Murata M, Handa M, Ikeda Y (2002) Reconstitution of adhesive properties of human platelets in liposomes carrying both recombinant glycoproteins Ia/IIa and Ib alpha under flow conditions: specific synergy of receptor-ligand interactions. Blood 100(1):136–142
Palacios-Acedo AL, Mege D, Crescence L, Dignat-George F, Dubois C, Panicot-Dubois L (2019) Platelets, thrombo-inflammation, and cancer: collaborating with the enemy. Front Immunol 10:1805
Zhu XH, Du JX, Zhu D, Ren SZ, Chen K, Zhu HL (2020) Recent research on methods to improve tumor hypoxia environment. Oxid Med Cell Longev 2020:5721258
Squires JE (2002) Artificial blood. Science 295(5557):1002–1005
Thiagarajan P, Parker CJ, Prchal JT (2021) How do red blood cells die? Front Physiol 12:655393
Kresie L (2001) Artificial blood: an update on current red cell and platelet substitutes. Proceedings 14(2):158–161
Marks DH, Brown DR, Ottinger WE, Atassi MZ (1987) Antibody response to transfusion with pyridoxalated polymerized hemoglobin solution. Mil Med 152(9):473–477
Fleming P, Townsend E, Van Hilten JA, Spence A, Ferguson E (2012) Expert relevance and the use of context-driven heuristic processes in risk perception. J Risk Res 15(7):857–873
Chen J-Y, Scerbo M, Kramer G (2009) A review of blood substitutes: examining the history, clinical trial results, and ethics of hemoglobin-based oxygen carriers. Clinics 64:803–813
Fleming P, Ferguson E, Townsend E, Lowe KC (2007) Perceptions in transfusion medicine: a pilot field study on risk and ethics for blood and blood substitutes. Artif Cells Blood Substit Biotechnol 35(2):149–156
Crowe EP, DeSimone RA (2022) When blood transfusion is not an option owing to religious beliefs. Annals of Blood 7:6723
Schumacher A, Sikov WM, Quesenberry MI, Safran H, Khurshid H, Mitchell KM et al (2017) Informed consent in oncology clinical trials: a Brown University Oncology Research Group prospective cross-sectional pilot study. PLoS ONE 12(2):e0172957
Lo B, Parham L (2009) Ethical issues in stem cell research. Endocr Rev 30(3):204–213
Trials of war criminals before the Nuremberg Military Tribunals under Control Council Law No. 10
Sawyer C, Preston L, Taylor S, Davies M, Carter L, Krebs M et al (2021) Oncology patients’ experiences in experimental medicine cancer trials: a qualitative study. BMJ Open 11(10):e047813
Chen JY, Scerbo M, Kramer G (2009) A review of blood substitutes: examining the history, clinical trial results, and ethics of hemoglobin-based oxygen carriers. Clinics (Sao Paulo) 64(8):803–813
Satyanarayana Rao KH (2008) Informed consent: an ethical obligation or legal compulsion? J Cutan Aesthet Surg 1(1):33–35
Kadam RA (2017) Informed consent process: a step further towards making it meaningful! Perspect Clin Res 8(3):107–112
Marrazzo F, Larson G, Sherpa Lama TT, Teggia Droghi M, Joyce M, Ichinose F et al (2019) Inhaled nitric oxide prevents systemic and pulmonary vasoconstriction due to hemoglobin-based oxygen carrier infusion: a case report. J Crit Care 51:213–216
Zhu K, Wang L, Xiao Y, Zhang X, You G, Chen Y et al (2024) Nanomaterial-related hemoglobin-based oxygen carriers, with emphasis on liposome and nano-capsules, for biomedical applications: current status and future perspectives. J Nanobiotechnol 22(1):336
Alayash AI (2019) Mechanisms of toxicity and modulation of hemoglobin-based oxygen carriers. Shock 52(1S Suppl 1):41–49
Nagababu E, Rifkind JM (2000) Heme degradation during autoxidation of oxyhemoglobin. Biochem Biophys Res Commun 273(3):839–845
Premont RT, Reynolds JD, Zhang R, Stamler JS (2020) Role of nitric oxide carried by hemoglobin in cardiovascular physiology: developments on a three-gas respiratory cycle. Circ Res 126(1):129–158
Sen Gupta A (2019) Hemoglobin-based oxygen carriers: current state-of-the-art and novel molecules. Shock. 52:70–83
Taguchi K, Yamasaki K, Maruyama T, Otagiri M (2017) Comparison of the pharmacokinetic properties of hemoglobin-based oxygen carriers. J Funct Biomater 8(1):11
Stephens RW, Orning L, Stormorken H, Hamers MJ, Petersen LB, Sakariassen KS (1996) Characterisation of cell-surface procoagulant activities using a microcarrier model. Thromb Res 84(6):453–461
Saha D, Patgaonkar M, Shroff A, Ayyar K, Bashir T, Reddy KV (2014) Hemoglobin expression in nonerythroid cells: novel or ubiquitous? Int J Inflamm 2014:803237
Fiorito V, Chiabrando D, Petrillo S, Bertino F, Tolosano E (2019) The multifaceted role of heme in cancer. Front Oncol 9:1540
Kruczkowska W, Kciuk M, Pasieka Z, Klosinski K, Pluciennik E, Elmer J et al (2023) The artificial oxygen carrier erythrocruorin-characteristics and potential significance in medicine. J Mol Med 101(8):961–972
Creteur J, Vincent JL (2009) Potential uses of hemoglobin-based oxygen carriers in critical care medicine. Crit Care Clin 25(2):311–24
Liu YP, Zheng CC, Huang YN, He ML, Xu WW, Li B (2021) Molecular mechanisms of chemo- and radiotherapy resistance and the potential implications for cancer treatment. MedComm 2(3):315–340
Acknowledgements
All the figures in the manuscript have been generated using BioRender.com.
Funding
This research was supported by funding in the form of an extramural grant (Grant # 5/13/55/2020/NCD-III) from the Indian Council of Medical Research (ICMR), Government of India, New Delhi, to MKK.
Author information
Authors and Affiliations
Contributions
MKK and LK designed and supervised the study. Substantial contribution is assigned to LK, MK, MKK, and RS in form of extensive literature review, writing and development of visual aids, including figures and tables. Additionally, the manuscript was critically edited and revised multiple times based on the input from HZ, LK, MK, MKK, and RS. All the authors read and approved the final version of the manuscript.
Corresponding authors
Ethics declarations
Ethics approval
This study involves review of literature and analysis and does not involve directly or indirectly any patient sample, cell line, or even patients or normal subjects for participation in the study.
Consent to participate
The study does not involve any human subjects, so ethical approval and consent to participate is not applicable.
Consent for publication
All the authors agree to the content of the manuscript and have no conflict of interest for authorship.
Competing interests
MKK has received consultant honoraria from the CBRS, Noida. The rest of the authors declare that they have no financial interests.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Sharma, R., Kashyap, M., Zayed, H. et al. Artificial blood—hope and the challenges to combat tumor hypoxia for anti-cancer therapy. Med Biol Eng Comput 63, 933–957 (2025). https://doi.org/10.1007/s11517-024-03233-6
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11517-024-03233-6