Abstract
This work aims at examining the impact of generated CO2 nanobubbles (NBs) via the membrane-based method on physicochemical properties and surface tension of commercial clarified apple juice. The gas was injected at 300 kPa pressure for variable liquid circulation times (5, 13 and 26 min) to produce the CO2 NBs. Sets of 13- and 26-min circulation time to mix CO2 and liquid gave the desirably nano-size (~ 80–200 nm) NBs and significantly (p ≤ 0.05) reduced surface tension (by ~ 20–25%) of the juice dispersed with these formed tiny gas bubbles (NB-juice). An increase in circulation time also resulted in more negative zeta potential and higher dissolved CO2 concentration of the NB-juice. Density values of apple juice remained unchanged with and without incorporating CO2 NBs. These experimental outcomes provide the potential use of NBs in controlling the characteristics of liquid food as an environment-friendly approach to minimise chemical usages.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Recently, exceptional features presented by ultrafine gas spheres known as nanobubbles (NBs) suspended in liquid medium have been suggested as a highly promising strategy for treating different challenges in various branches of science and technology [1, 2]. Some well-known applications of fine and ultrafine gas bubbles or micro- and nanobubbles (MNBs) include sanitisation and water treatment [3, 4], getting rid of pesticide [5], enhancing seed germination, growing of plants and fish [6, 7] and supporting medical researches for efficient drug delivery and release [8, 9]. Aside from commonly applied fields such as agriculture, biochemistry, and medical sciences, usages of gas NBs in food areas have also been attracting considerable attention due to some prominent beneficial characteristics offered by NBs to food materials.
With a size range of several hundred nanometers [1, 10], NBs display unique properties against their macro-scaled counterparts [2, 11, 12]. As a case in point, high internal pressure and extremely tiny diameter of NBs imply a huge surface area/volume ratio, allowing to improve intensiveness of mass transfer, which are expected in various food processes [13, 14]. In addition, remarkably high solubility of gas under nanobubble (NB) form [7, 15] and unusual stability of NBs against coalescence [4, 16] are also extraordinary aspects resulting in many benefits on applying them in either laboratory research or industrial scale. The pre-combination of O2 MNBs was found to enhance the ultrasound cavitation, assisting the ice nucleation and crystallisation during freezing process of sucrose and maltodextrin solutions [15]. Amamcharla, Li and Liu [17] and Phan, Truong, Wang and Bhandari [14] found that the existence of bulk gas NBs from the air, O2, or CO2 could significantly decrease viscosities of food fluids by retarding the aggregation of suspended particles as well as playing as a buffer or diluting agent in the food systems.
Along with viscosity, surface tension has a vital role in food processes [14, 18]. For instance, the properties of maltodextrin and whole milk powder, such as wettability, dispersibility and solubility in liquid, depended on the liquid surface tension [19]. The surface tension of water also had a direct effect on the rate of homogeneous and heterogeneous nucleation in water crystallisation relating to freezing foods [20]. Regarding colloidal solutions, surface tension is one of the key parameters required to govern and predict the formation and physical stability of emulsions [21]. Very few attempts have been made to investigate the impact of NBs on the surface tension of liquid foods. A decrease by 10% of surface tension value of water mixed with ozone (O3) ultrafine bubbles (diameter range of 10–500 nm) was observed by Ushida, Koyama, Nakamoto, Narumi, Sato and Hasegawa [22]. Our previous study obtained the high effectiveness of CO2 NBs (~ 30–100 nm) in lowering the surface tension of water containing sodium dodecyl sulfate (SDS) compared with the control sample (SDS solution having no CO2 NBs) [23]. Both samples contained SDS at thousand times lower content than its critical micelle concentration (CMC). Apart from these, the investigations and understanding about applications of NBs in food areas are infancy, and the influence of NBs suspension on properties of liquid foods is still limited. Accordingly, it is well worth considering the potentials and advantages of gaseous NBs, as an alternative means to chemical utilisations, in controlling food fluid characteristics.
In this research, we present the generation of bulk CO2 NBs in distilled (DI) water, and in a commercial clarified apple juice by utilising the NB generator designed with a membrane module. The formed bubbles’ characteristics consisting of size distribution, size values and zeta potential (ZP) were evaluated. We also examined the surface tension and other physicochemical properties, including density, pH and dissolved CO2 content of the fruit juice injecting with the gas NBs (NB-juice) during the storage period (7 days) as against the control samples (apple juice without any CO2 treatment). Besides, discussion to get further understanding about the effectiveness, as well as promising prospects of using NBs on controlling behaviour of liquid food medium, is presented.
Materials and Methods
Materials
The commercial Golden Circle apple juice was purchased from a local chain of supermarket (Brisbane, QLD, Australia). The clarified apple juice was employed as the medium to generate NBs. The juice clarity allowed to reduce the interference from the existing components in the NB properties determination.
NB-generator System
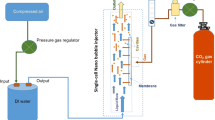
The schematic diagram of membrane-based circulation system to generate bulk gas NBs in liquid medium is illustrated in Fig. 1. Details about the setup of the designed NB-generator are specified in the research work of Phan, Truong, Wang and Bhandari [14]. To create the bulk NBs dispersed in an aqueous phase, the gas from a CO2 gas cylinder (food-grade CO2, purity > 99.5%, Coregas Co., Australia) was firstly injected at the tested level through the nano-sized pores (at 50 nm) of a ceramic membrane tube (TIA Co., Bollene, France), housed in a membrane cartridge. The external and internal diameters of the applied membrane were 10 and 8 mm, respectively, and the length was 250 mm. After the gas introduction, the liquid phase from a reservoir tank was conveyed and circulated by a pump (17 L/min, 12 V pressure pump DC, Kickass Products Pty Ltd., Australia) and attached liquid lines around the membrane module, as depicted in Fig. 1. Inside the membrane cartridge, there were two rubber rings controlled the flow of inlet and outlet liquid, so that the solution would only be transferred within the interior of the membrane tube. The mechanism for the formation or detachment of NBs from the membrane surface is due to the shear stress caused by the pressurisation of gas injected via extremely small membrane nano-pores into the moving aqueous medium [4, 11, 14, 23].
Generation and Visualisation of CO2 NBs Formed in Water
Before introducing bulk CO2 NBs into apple juice, preliminary tests to determine the optimum set of parameters, including injection gas pressure and circulation time for generating the gas NBs in pure laboratory DI water, were performed by employing the response surface methodology. Results depicted in Fig. 2 show that the optimum conditions were 300 kPa of gas pressure together with 13 min of liquid circulation time around the membrane cartridge of the generator system. By applying the optimum set, the water medium showed a highly dissolved CO2 content of 2000–2200 ppm (by mass) with bulk CO2 NBs of around 300–350 nm in size.
Schematic of circulation nanobubble-generator system designed based on membrane technique (adapted and modified from Phan, Truong, Wang and Bhandari [14])
Cryogenic scanning electron microscopy (Cryo-SEM) (JSM-7100 F, JEOL Ltd., Japan) furnished with a Gatan low temperature section was used to capture the presence of formed NBs in DI water, as described in our previous work [23]. The Cryo-SEM sample preparation involved in the quenching copper holder, which contained approximately 1 µL of the water sample dispersing CO2 NBs, into a liquid nitrogen cup (~-180oC). The specimens were then placed in a prepared cold chamber (with liquid nitrogen) and fractured using a pre-frigid scalpel blade. Afterwards, they were engraved at -90oC for 10 min and coated with platinum before taking images at 2 kV of voltage (to decrease the fluctuation of temperature) in the cold stage of Cryo-SEM system. Liquid nitrogen was transferred periodically into the cooling chamber to maintain the required low temperature of visualisation process.
Generation of CO2 NBs in Apple Juice
Relying on the optimum generation conditions to produce ultrafine CO2 bubbles at nanosized scale in DI water, the sets by combining 300 kPa of injection gas pressure with three different circulation times including 5, 13 and 26 min were chosen to test the formation of CO2 gas NBs in apple juice (NBAJ). The selected sets are detailed and coded in Table 1.
After generation in the juice medium, features (size distribution, size values and ZP) of generated CO2 NBs and attributes (surface tension, density, pH, and dissolved CO2 amount) of apple juice dispersed with them (NB-juice) were measured right away, as well as during the period of their retention (7 days). The samples containing NBs were stored in sterilised containers with un-tight caps at ambient temperature (23 ± 2oC). The controls, which were apple juice samples without the gas treatment and stored at the same condition, were also evaluated, and compared the characteristics with the NB-juices according to the storage time.
Characteristics of Generated CO2 NBs in Apple Juice
Measurement of Size Distribution
Size distribution density of CO2 NBs suspended in apple juice was evaluated relying on scattering and diffraction of laser light source on the NBs using dynamic light scattering (DLS) technique of the Zetasizer (Zetasizer Nano ZS, Malvern Panalytical Ltd., UK) following to Phan, Truong, Wang and Bhandari [14] and Wang, Zhao, Qi, Qin, Zhang and Li [24]. Number size distribution values including Dn0.5 and Dn0.9, which are 50%, 90% of bubble population undersize, were also determined.
Measurement of ZP
The Zetasizer (Zetasizer Nano ZS, Malvern Panalytical Ltd., UK) was also employed to measure ZP values of NBs dispersed in NBAJ samples and the control. Measurements were performed in U-shape cell (DTS1060) at 25oC by applying the laser doppler velocimetry technique and calculated by the Smoluchowski equation. Further details about the measurement protocols can be found in the recently published works [11, 23, 24].
Physicochemical Characteristics of Apple Juice Dispersed with CO2 NBs
Determination of Density and pH Values
Density and pH values of apple juice samples injected with CO2 NBs and their controls were directly determined using calibrated pycnometers (Labtek Pty Ltd., QLD, Australia) and a pH measuring instrument (pH Cube Benchtop pH-mV, TPS Pty Ltd., Australia), respectively, at ambient temperature (23 ± 2oC) [14].
Determination of Dissolved CO2 Amount
Solubilised CO2 concentrations of treated apple juice samples and the control during storage time were evaluated using the titration method as described by Truong, Palmer, Bansal, Bhandari and Hub [25] and Phan, Truong, Wang and Bhandari [14]. There were two 100 mL Buchner flasks connected with each other by a neoprene tubing. One flask (flask A) contained 5 mL of H2SO4 0.5 M, while the other (flask B) hold 3 mL of standardised Ba(OH)2 0.05 M. When the set of Buchner flasks was ready, 2 g of juice sample was put into the flask carrying H2SO4 0.5 M, then the flask-set would be closed by conical rubber stoppers for 18–24 h at ambient temperature (23 ± 2oC) for equilibrating. During the equilibration time, there would be the reaction between the evolved CO2 from sample and Ba(OH)2 (in flask B) leading to the precipitation of BaCO3. Afterwards, the remained concentration of Ba(OH)2 in flask B was determined by titration with HCl 0.1 M using phenolphthalein as the indicator. Three replications were applied for each sample.
Determination of Surface Tension
The surface tension of apple juice samples dispersed with CO2 NBs and the controls were measured by the Kruss force tensiometer (K11, Kruss GmbH, Hamburg, Germany) with the force transducer accuracy of 0.1 mN/m. The Wilhelmy plate method was employed to quantify the force acting on the Kruss standard plate (19 × 10 × 0.2 mm) immersed into the liquid medium as described by Nardello-Rataj and Leclercq [26] and Phan, Truong, Wang and Bhandari [23]. Numerous measurements were conducted at linear intervals for each sample until obtaining the equilibrium value of surface tension. There were also other setting parameters which are surface detection speed (10 mm/min), immersion depth (2 mm) and surface detection sensitive (0.01 g) used for the evaluation of surface tension. The flame of Bunsen burner was applied to rinse off the platinum plate before each measurement. All samples were measured at least three times.
Statistical Analysis
The Minitab statistical package (Minitab release 17, Minitab Inc., Stage College, PA, USA) was used for experimental data analysis. Mean values of replications were calculated and presented with standard deviation (± SD). One-way analysis of variance (ANOVA) and Turkey’s post hoc test were applied to evaluate the significant (at p ≤ 0.05) differences among treatments and the controls.
Results and Discussion
Micrographs of Bulk CO2 NBs Dispersed in DI Water
The representative Cryo-SEM images of bulk CO2 NBs generated in DI water and that of the water control without the gas injection are shown in Fig. 3. One can clearly find that there are emptily spherical holes at around 100–300 nm of diameter located on the ice surface of water sample containing CO2 NBs, as shown in Fig. 3a-c. Whereas, the image capture of control water (Fig. 3d) with no CO2 treatment and at the same magnification has the smoother ice-face without the hollow hole. Accordingly, the generation of bulk CO2 NBs in aqueous medium can be confirmed, and this also means that the formation of these tiny gas-filled cavities in other food liquid is possible. In addition, among obtained images of CO2 NBs by means of Cryo-SEM, the case observed in Fig. 3c with a larger and un-round hole demonstrates the possibility of bubble coalescence and/or agglomeration because of unfavourable effects occurring during freezing process of the visualisation technique [23, 27].
Characteristics of CO2 NBs Generated in Apple Juice
Size Distribution and size Values
Figure 4a illustrates the number size distribution of CO2 gas NBs right after production in apple juice using the injection gas pressure at 300 kPa with three different circulation times (5, 13 or 26 min) and the control. Likewise, the changes in size range of dispersed NBs in juice samples after storage periods of 1, 3 and 7 days versus their controls are plotted in Fig. 4b-d.
Firstly, there was a clear difference in NBs’ size distribution between the treated NB-juice samples (~ 80–800 nm) and their controls (~ 5–10 nm) at any monitored time point after bubble generation. Additionally, as shown in Fig. 4, size distribution of the controls seems to be unvaried during retention time. This size range is comparatively similar with the molecular radius of chemical compounds existing in plants/fruits such as polysaccharides, pectin, polyphenols, and proteins [28, 29]. The size distribution measurements in Fig. 4 also show that size ranges of generated NBs decrease accordingly with the rise in applied circulation time of mixing liquid and gas. Moreover, there is an increase in size distribution of NBs of all NB-juice samples following the duration of storage, especially those of NBAJ-5 min, the shortest time used for circulation of NB generation. NBs can collapse, coalesce, or experience Ostwald ripening after formation, as were previously reported by Ahmed, Sun, Hua, Zhang, Zhang, Zhang and Marhaba [16] and Phan, Truong, Wang and Bhandari [11]. Nevertheless, despite after 7 days of generation, the size distribution curves of all gas treatments to generate NBs in apple juice were still observed in the range of several hundred nanometers (lower than 1000 nm) and maintained the good mono-modal shape. Long-term stability of gas NBs could be attributed to their unique structure with hydrogen bonding network and the covering double charge layer equilibrating with the Laplace high internal pressure, which reduce gas diffusivity through NB-liquid interface to prevent bubble coalescence and shrinkage [1, 2]. Recently, Niwano, Ma, Iwata, Tadaki, Yamamoto, Kimura and Hirano-Iwata [30] reported that there were three, four- and five-membered ring clusters of water molecules observed at the interface structure of bulk gas NBs by using infrared reflection–absorption (IRAS) and nuclear magnetic resonance (NMR) spectroscopy techniques. In these ring clusters, molecules of water are bound more tightly than those in free water medium, and also single clusters are bound together in the interfacial layer which is similar to the solid state of water (i.e., ice layer) or a hydrogen bonding network, contributing significantly into the stability of tiny gas NBs [30]. Besides, the supersaturated gas level of surroundings, which would be presented in the later part, can also be another factor supporting the longevity of bulk NBs. Observations in the research work of Ushikubo, Furukawa, Nakagawa, Enari, Makino, Kawagoe, Shiina and Oshita [12] indicated that the small gradient concentration of gas between NBs and the bulk medium due to a high amount of dissolved gas in liquid around NBs corroborates with the possible stability of them since the gas diffusion at bubble interface would take longer time.
Determined size values of generated CO2 NBs include Dn0.5 and Dn0.9 following the storage time are illustrated in Fig. 5. Dn0.5 and Dn0.9 size values of NBs grow respectively from 70 to 400 nm and from 90 to 600 nm after 7 days. The growth rate of size values is also higher with the shorter circulation time used to generate the NBs. Oh and Kim [31] and Phan, Truong, Wang and Bhandari [14] reported the same effect of mixing and circulation time on size of bulk CO2 NBs in different liquids. The more stable NBs having smaller mode diameters were found with an increase in circulation time to infuse the gas into liquid. Studies on the production of bulk NBs by applying membrane technique have also demonstrated that over a long mixing period, larger gaseous cavities would escape from the medium leaving the smaller ones left due to the impact of buoyancy force [14, 16].
Size values of CO2 NBs dispersed in apple juice just after generation and during storage time of 7 days. For each observed size value, Dn0.5 or Dn0.9, the means (± SD) sharing the same upper-case letter at the same circulation time treatment (impact of storage period) or the same lower-case letter at the same monitored time including just after generation, 1, 3 or 7 days (impact of circulation time treatment) were not significantly (p > 0.05) different
Zeta Potential (ZP)
The experimental results about ZP of CO2 NBs generated in apple juice are displayed in Fig. 6a. There is a positive correlation between the magnitude of ZP and the circulation time to form the ultrafine gas bubbles. The more negative ZP values of NBs of NBAJ-13 min and NBAJ-26 min are in accordance with the smaller in their number size distribution and the slower growth of their size values over days of retention. The same observations were stated in published research by Ahmed, Sun, Hua, Zhang, Zhang, Zhang and Marhaba [16] and Phan, Truong, Wang and Bhandari [11]. It has been known that NBs are negatively charged owing to the excess OH− ions comparative to H+ ions at the gas and water interface of bubbles [1, 4, 32]. The absolute ZP values of air, N2 and O2 NBs were 17–20, 29–35 and 34–45 mV, respectively, as reported by Ushikubo, Enari, Furukawa, Nakagawa, Makino, Kawagoe and Oshita [32]. In this study, the magnitudes of ZP of CO2 NBs formed in apple juice samples are lower (in the range of 9–15 mV). This can be explained by the lower pH value of the apple juice (< 3.5, which would be discussed in the following section) resulted from the existence of organic acid compounds [33] and the dissolution of CO2 in the juice increasing the H+ ions. These positive ions would be absorbed onto the bubble-liquid interface leading to a drop in the number of OH− group or the less negative ZP of the generated CO2 NBs [11, 32, 34].
Zeta potential (ZP) of generated CO2 NBs (A); and density values of apple juice dispersed the NBs (B) generated by using different treatments (coded and explained in Table 1). Controls were those of the juice samples without CO2 treatment. ZP and density values sharing the same upper-case and lower-case letter, respectively, were not significantly (p > 0.05) different
Properties of Apple Juice Dispersed CO2 NBs
Density, Dissolved CO2 Concentration and pH Values
The results of density of apple juice injected with CO2 NBs are presented in Fig. 6b. There is no significant (p > 0.05) difference in density among the juice samples with and without the presence of CO2 NBs. Our earlier research on the same gas NBs formed in fruit juice concentrate and vegetable oil also found the same tendency indicating that incorporation of these ultrafine bubbles causes no propounded effect on the density of the medium [14].
Dissolved concentration of CO2 in apple juice dispersed with the gas nanobubbles (NBs) generated by using 300 kPa of gas pressure and three different circulation times (5, 13, and 26 min), and the control without CO2 injection during storage period of 7 days at ambient temperature (23 ± 2oC). At the same retention time, the treatment sharing the same upper-case letter had no significant (p > 0.05) difference. Under the same treatment, storage date sharing the same lower-case letter had no significant (p > 0.05) difference
Concentrations of CO2 dissolved in NBAJ-5 min, NBAJ-13 min, NBAJ-26 min, and their controls following the storage time are presented in Fig. 7. As shown in the graph, the higher amount of solubilised CO2 of NB-juice is related to the longer circulation time applied to generate them. During the storage period, although amounts of the dissolved gas in all juice samples injected with CO2 NBs are gradually reduced, their concentrations are still significantly (p ≤ 0.05) higher than the controls stored at the same condition. Additionally, as depicted in Fig. 7, the measured levels of dissolved CO2 in NB-juice subjected to 13 and 26 min of circulation time are more than that of the NBAJ-5 min over the monitored time. There are also significantly (p ≤ 0.05) lower size values and size distribution in number (mentioned above) of NBAJ-13 min and NBAJ-26 min. Studies have shown that stability of the small size scale of gas NBs can be supported and prolonged by maintaining the high level of dissolved gas in the liquid [12, 14, 22].
The determined data of pH of apple juice incorporated with CO2 NBs and those of the control during the storage period are compared in Table 2. Firstly, the difference in pH values between just generated NB-juice and the control is marginal. The findings can be acknowledged by the fact that pH of apple juice is already low due to the richness in acidic compounds like malic, tartaric and citric acids [33]. Nevertheless, within 7 days of storage at ambient temperature (23 ± 2oC), the pH values undergo an upward trend for all treatments (Table 2), and there is a significantly (p ≤ 0.05) lower in pH value of NBAJ-13 min and NBAJ-26 min than the others. The increase in pH of fruit juices over retention time can be explained by considering the loss in acidic content of the juices [35, 36]. However, the less rise in pH values of NB-juice generated with 13 and 26 min of circulation time appears to be compromised with the high amount of solubilised CO2 maintained in the NB treated samples during days of storage at 23 ± 2oC.
At the same monitored time (same column), the average (± SD) value of pH sharing the same letter had no significant (p > 0.05) difference.
Surface Tension
Plots of surface tension measured by Welhelmy method of NB-juice samples and the control just after their generation and during 7 days of retention are shown in Fig. 8. There are significant (p ≤ 0.05) declines in surface tension values of NBAJ-13 min (58.4 mN/m) and NBAJ-26 min (54.4 mN/m) samples when comparing with the control (without the gas treatment; 72.9 mN/m). The discrepancy in surface tension values between NB treated juice and the control is maintained during the storage time and positively correlated with the longer circulation time applied to produce CO2 NBs. These NB treatments coded as NBAJ-13 min and NBAJ-26 min also gave smaller size ranges, size values, and more negative ZP of formed NBs as mentioned above. Furthermore, they had higher dissolved CO2 concentration, lower pH and the slower changes in these characteristics as compared with those of the NB-juice generated with shorter applied circulation time (NBAJ-5 min).
Surface tension of apple juice injected with bulk CO2 NBs during storage: (A) just after NBs generation; (B) after 1 day; (C) after 3 days; and (D) after 7 days. The controls are the juice samples without CO2 treatment. All treated samples and their controls were stored and observed at the same condition and period of retention
The decrease in surface tension in the apple juice containing CO2 NBs agrees with recent findings on ultrasonic water having O2 NBs. According to Lee, Yim and Kim [37], the dissolved O2 content in the ultrasonic water existing with the gas NBs was significantly increased. They also found that the supersaturated level of O2 gas in DI water caused a reduction in surface tension of the aqueous solution from 73 mN/m to 68 mN/m. The influence of the gas NBs on the drop in surface tension can be attributed to the role of the dissolved gas, which might become a surfactant reducing the attraction force among water molecules of the system [37]. Lubetkin [38] also suggested that surface tension of a liquid with and without dissolved gas is unalike. Fukuzawa, Watanabe, Yasuda and Ohmura [39] also demonstrated that there was a significant decrease in the interfacial tension of water because of the adsorption of CO2 and H2 gases at high pressure causing the hydrate formation. In another work, the presence of CO2 NBs in a low concentration solution of SDS (an anionic solution) showed that there was a significant decrease in surface tension by approximately 12% with the incorporation of NBs as against the control having the same amount of substance [23]. The mixing of ultrafine O3 bubbles into water also led to 10% less static surface tension than the one of water alone [22]. Using the same Wilhelmy technique to determine surface tension of liquid, Wolert, Setz, Underhill and Duran [40] presented that a liquid film would be more easily ruptured if there were air NBs existed at the liquid film surface. The possibility can be the rupture of liquid film occurring with the burst and disappearance of NBs at the air-liquid interface, resulting in the decrease in evaluated value of surface tension [40, 41]. Experiments carried out by Ushida, Hasegawa, Takahashi, Nakajima, Murao, Narumi and Uchiyama [42] also observed a reduction in surface tension of liquid water from about 73 mN/m to 63 mN/m with the presence of bulk air NBs. Besides, these authors found that there was a considerable decrease in surface tension of an anionic surfactant solution injected NBs, while the surface tension of solution containing a cation surfactant and same gas NBs remained unchanged. It was assumed that the local hydrophobicity of liquid system is more concentrated with the presence of negatively charged NBs possessing some hydrophobic parts at the bubble surface [41, 42]. In this research work, apple juice which would contain some proteins and phenolic compounds [43], was used as the liquid phase for bulk CO2 NBs generation. Thus, possible interactions between these negative charged groups and the dispersed CO2 NBs can occur, increasing the hydrophobicity of the whole liquid system, or reducing the surface tension of the juice due to the presence of CO2 NBs.
Surface tension plays a critical role in formulating, processing and testing quality of different food industrial products [44, 45]. Reducing surface and interfacial tension is one of key steps to mix two immicible liquids with each other as well as stabilise these interfaces for variably applying in food colloidal and foam systems [46, 47]. A decrease in interfacial tension also brings advantages by reducing the amount of mechanical work required to make emulsions or foams [48]. Rahman [49] observed that lowering surface tension allowed to decrease the capillary collapse in some cases of drying foods. By lowering the surface tension of chitosan coating solution, there was an enhancement in wettability of the liquid and also a denser coating film on the Fuji apple skins [50]. In addition, controlling the surface tension supported in countering the adhesion of liquid food to packaging/equipment surfaces, then significantly increased economic benefits [51]. Applying food-grade gas NBs would offer a promising and green solution as a possible alternative to minimise the usages of chemicals or surface-active compounds to govern surface and/or interfacial tension properties of food fluids.
Conclusion
This research work carried out the generation of bulk CO2 NBs in a real liquid food using a scalable circulation NB-generator integrated with the ceramic membrane module. The obtained results showed that the size and ZP of formed CO2 NBs in apple juice were dependent on the time of circulation to mix gas and liquid under the same injection gas pressure. The higher in the length of applied circulation period (up to 26 min) offered the smaller in size range of formed NBs as well as increasing their stability. NB-juice samples produced by these conditions also possessed higher dissolved CO2 concentration, lower pH and significantly lower values of surface tension against their control and the shorter time treatment (NBAJ-5 min). There was no change in the density of apple juice with the presence of CO2 NBs. Our findings could be used to verify the formation and longevity of food-grade gas NBs in food fluids as well as their possible influence on the physicochemical properties of the food medium incorporated them. Moreover, we believe that further understanding the promising effect of NBs in controlling liquid surface tension without using chemical surfactants could bring lots of practical applications and remarkable benefits to food and beverages field, in terms of processability, scalability and cost-efficiency. Further research works should be performed to detect and elucidate the potentials of incorporation of ultrafine gas NBs into different complex food systems.
Data Availability
All data generated or analyzed during this study are included in this published article.
References
K. Phan, T. Truong, Y. Wang, B. Bhandari, Nanobubbles: fundamental characteristics and applications in food processing. Trends Food Sci. Technol. 95, 118–130 (2020)
K. Ulatowski, P. Sobieszuk, Gas nanobubble dispersions as the important agent in environmental processes – generation methods review. Water and Environment Journal. 34, 772–790 (2020)
T. Temesgen, T.T. Bui, M. Han, T.I. Kim, H. Park, Micro and nanobubble technologies as a new horizon for water-treatment techniques: a review. Adv. Colloid Interface Sci. 246, 40–51 (2017)
K. Ulatowski, P. Sobieszuk, A. Mróz, T. Ciach, Stability of nanobubbles generated in water using porous membrane system. Chem. Eng. Process. - Process Intensif. 136, 62–71 (2019)
H. Ikeura, F. Kobayashi, M. Tamaki, Removal of residual pesticide, fenitrothion, in vegetables by using ozone microbubbles generated by different methods. J. Food Eng. 103, 345–349 (2011)
K. Ebina, K. Shi, M. Hirao, J. Hashimoto, Y. Kawato, S. Kaneshiro, T. Morimoto, K. Koizumi, H. Yoshikawa, Oxygen and air nanobubble water solution promote the growth of plants, fishes, and mice. PLoS One. 8, e65339 (2013)
S. Liu, Y. Kawagoe, Y. Makino, S. Oshita, Effects of nanobubbles on the physicochemical properties of water: the basis for peculiar properties of water containing nanobubbles. Chem. Eng. Sci. 93, 250–256 (2013)
T.M. Krupka, L. Solorio, R.E. Wilson, H. Wu, N. Azar, A.A. Exner, Formulation and characterization of echogenic lipid-pluronic nanobubbles. Mol. Pharm. 7, 49–59 (2010)
T. Noguchi, K. Ebina, M. Hirao, T. Morimoto, K. Koizumi, K. Kitaguchi, H. Matsuoka, T. Iwahashi, H. Yoshikawa, Oxygen ultra-fine bubbles water administration prevents bone loss of glucocorticoid-induced osteoporosis in mice by suppressing osteoclast differentiation. Osteoporos. Int. 28, 1063–1075 (2017)
T. Uchida, S. Oshita, M. Ohmori, T. Tsuno, K. Soejima, S. Shinozaki, Y. Take, K. Mitsuda, Transmission electron microscopic observations of nanobubbles and their capture of impurities in wastewater. Nanoscale Res. Lett. 6(295), 2–9 (2011)
K. Phan, T. Truong, Y. Wang, B. Bhandari, Formation and Stability of Carbon Dioxide Nanobubbles for potential applications in Food Processing. Food Eng. Rev. 13, 3–14 (2020)
F.Y. Ushikubo, T. Furukawa, R. Nakagawa, M. Enari, Y. Makino, Y. Kawagoe, T. Shiina, S. Oshita, Evidence of the existence and the stability of nano-bubbles in water. Colloids Surf., a 361, 31–37 (2010)
A. Ghadimkhani, W. Zhang, T. Marhaba, Ceramic Membrane Defouling (Cleaning) by air Nano Bubbles, vol. 146 (Chemosphere, 2016), pp. 379–384
K. Phan, T. Truong, Y. Wang, B. Bhandari, Effect of CO2 nanobubbles incorporation on the viscosity reduction of fruit juice concentrate and vegetable oil. Int. J. Food Sci. Technol. 56, 4278–4286 (2021)
Z. Zhu, D.W. Sun, Z. Zhang, Y. Li, L. Cheng, Effects of micro-nano bubbles on the nucleation and crystal growth of sucrose and maltodextrin solutions during ultrasound-assisted freezing process. LWT - Food Science and Technology. 92, 404–411 (2018)
A.K.A. Ahmed, C. Sun, L. Hua, Z. Zhang, Y. Zhang, W. Zhang, T. Marhaba, Generation of nanobubbles by ceramic membrane filters: the dependence of bubble size and zeta potential on surface coating, pore size and injected gas pressure. Chemosphere. 203, 327–335 (2018)
J. Amamcharla, B. Li, Z. Liu, Use of micro- and nano-bubbles in liquid processing, United States, 2017
B. Adhikari, T. Howes, A. Shrestha, B.R. Bhandari, Effect of surface tension and viscosity on the surface stickiness of carbohydrate and protein solutions. J. Food Eng. 79, 1136–1143 (2007)
W.R. Mitchell, L. Forny, T. Althaus, G. Niederreiter, S. Palzer, M.J. Hounslow, A.D. Salman, Surface tension-driven effects in the reconstitution of food powders. Chem. Eng. Res. Des. 146, 464–469 (2019)
H. Kiani, D.-W. Sun, Water crystallization and its importance to freezing of foods: a review. Trends Food Sci. Technol. 22, 407–426 (2011)
P. Wilde, A. Mackie, F. Husband, P. Gunning, V. Morris, Proteins and emulsifiers at liquid interfaces, Adv. Colloid Interface Sci., 108–109 (2004) 63–71
A. Ushida, T. Koyama, Y. Nakamoto, T. Narumi, T. Sato, T. Hasegawa, Antimicrobial effectiveness of ultra-fine ozone-rich bubble mixtures for fresh vegetables using an alternating flow. J. Food Eng. 206, 48–56 (2017)
K. Phan, T. Truong, Y. Wang, B. Bhandari, Effect of electrolytes and surfactants on generation and longevity of carbon dioxide nanobubbles. Food Chem. 363, 130299 (2021)
Q. Wang, H. Zhao, N. Qi, Y. Qin, X. Zhang, Y. Li, Generation and Stability of size-adjustable bulk nanobubbles based on periodic pressure change. Sci. Rep. 9, 1118 (2019)
T. Truong, M. Palmer, N. Bansal, B. Bhandari, A.D.I. Hub, Effects of dissolved carbon dioxide in fat phase of cream on manufacturing and physical properties of butter. J. Food Eng. 226, 9–21 (2018)
V. Nardello-Rataj, L. Leclercq, Aqueous solutions of didecyldimethylammonium chloride and octaethylene glycol monododecyl ether: toward synergistic formulations against enveloped viruses. Int. J. Pharm. 511, 550–559 (2016)
A.J. Jadhav, M. Barigou, Bulk Nanobubbles or Not Nanobubbles: That is the Question, Langmuir, 36 (2020) 1699–1708
D. Gawkowska, J. Cybulska, A. Zdunek, Structure-related gelling of pectins and linking with other Natural Compounds: a review, Polym. (Basel), 10 (2018)
Q. Han, Q.-. Yu, J. Shi, C.-. Xiong, Z.-. Ling, -m. he, molecular characterization and hypoglycemic activity of a novel water-soluble polysaccharide from tea (Camellia sinensis) flower. Carbohydr. Polym. 86, 797–805 (2011)
M. Niwano, T. Ma, K. Iwata, D. Tadaki, H. Yamamoto, Y. Kimura, A. Hirano-Iwata, Two-dimensional water-molecule-cluster layers at nanobubble interfaces. J. Colloid Interface Sci. 652, 1775–1783 (2023)
S.H. Oh, J.M. Kim, Generation and Stability of Bulk Nanobubbles, Langmuir, 33 (2017) 3818–3823
F.Y. Ushikubo, M. Enari, T. Furukawa, R. Nakagawa, Y. Makino, Y. Kawagoe, S. Oshita, Zeta-potential of Micro- and/or Nano-bubbles in Water Produced by Some Kinds of Gases, IFAC Proceedings Volumes, 43 (2010) 283–288
Y. Huang, B.A. Rasco, A.G. Cavinato, Fruit Juices, Infrared Spectroscopy for Food Quality Analysis and Control2009, pp. 355–375
A. Mojaddar Langroodi, H. Tajik, T. Mehdizadeh, M. Moradi, E. Moghaddas Kia, A. Mahmoudian, Effects of sumac extract dipping and chitosan coating enriched with Zataria multiflora Boiss oil on the shelf-life of meat in modified atmosphere packaging, Lwt, 98 (2018) 372–380
B. Mgaya-Kilima, S.F. Remberg, B.E. Chove, T. Wicklund, Influence of storage temperature and time on the physicochemical and bioactive properties of roselle-fruit juice blends in plastic bottle. Food Sci. Nutr. 2, 181–191 (2014)
V. Kabasakalis, D. Siopidou, E. Moshatou, Ascorbic acid content of commercial fruit juices and its rate of loss upon storage. Food Chem. 70, 325–328 (2000)
J.I. Lee, B.S. Yim, J.M. Kim, Effect of dissolved-gas concentration on bulk nanobubbles generation using ultrasonication. Sci. Rep. 10, 18816 (2020)
S.D. Lubetkin, Why is it much easier to nucleate gas bubbles than theory predicts? Langmuir, 19 (2003) 2575–2587
K. Fukuzawa, K. Watanabe, K. Yasuda, R. Ohmura, Interfacial tension measurements in the (CO 2 + H 2) gas mixture and water system at temperatures from 271.2 K to 280.2 K and pressures up to 7.0 MPa. J. Chem. Thermodyn. 119, 20–25 (2018)
E. Wolert, S.M. Setz, R.S. Underhill, R.S. Duran, Meso- and microscopic behavior of spherical polymer particles assembling at the air-water interface. Langmuir. 17, 5671–5677 (2001)
K. Yasui, T. Tuziuti, N. Izu, W. Kanematsu, Is surface tension reduced by nanobubbles (ultrafine bubbles) generated by cavitation? Ultrason. Sonochem. 52, 13–18 (2018)
A. Ushida, T. Hasegawa, N. Takahashi, T. Nakajima, S. Murao, T. Narumi, H. Uchiyama, Effect of mixed nanobubble and Microbubble Liquids on the washing rate of Cloth in an Alternating Flow. J. Surfactants Deterg. 15, 695–702 (2012)
M. Cai, C. Xie, Y. Lv, K. Yang, P. Sun, Changes in physicochemical profiles and quality of apple juice treated by ultrafiltration and during its storage. Food Sci. Nutr. 8, 2913–2919 (2020)
K. Muthamizhi, P. Kalaichelvi, S.T. Powar, R. Jaishree, Investigation and modelling of surface tension of power-law fluids. RSC Adv. 4, 9771–9776 (2014)
R.S. Lam, M.T. Nickerson, Food proteins: a review on their emulsifying properties using a structure-function approach. Food Chem. 141, 975–984 (2013)
B.G. Ribeiro, J.M.C. Guerra, L.A. Sarubbo, Biosurfactants: production and application prospects in the food industry. Biotechnol. Prog. 36, e3030 (2020)
S.P. Eugénie, D. Fabrice, C. Gérard, M. Samir, Effect of bulk viscosity and surface tension kinetics on structure of foam generated at the pilot scale. Food Hydrocoll. 34, 104–111 (2014)
A.M. Bos, T.V. Vliet, Interfacial rheological properties of adsorbed protein layers and surfactants: a review. Adv. Colloid Interface Sci. 91, 437–471 (2001)
M.S. Rahman, Toward prediction of Porosity in Foods during drying: a brief review. Drying Technol. 19, 1–13 (2001)
W.Y. Choi, H.J. Park, D.J. Ahn, J. Lee, C.Y. Lee, Wettability of chitosan coating solution on ‘Fuji’ apple skin. Food Eng. Phys. Prop. 67, 2668–2672 (2002)
X. Liu, L. Wang, Y. Qiao, X. Sun, S. Ma, X. Cheng, W. Qi, W. Huang, Y. Li, Adhesion of liquid food to packaging surfaces: mechanisms, test methods, influencing factors and anti-adhesion methods. J. Food Eng. 228, 102–117 (2018)
Funding
This work was financially supported by the Australia-China Joint Research Centre in Future Dairy Manufacturing Dairy Innovation Centre - ACSRF48154 (2016–2020). The authors acknowledge the facilities, and the scientific and technical assistance, of the Microscopy Australia Facility at the Centre for Microscopy and Microanalysis (CMM), The University of Queensland (UQ). We appreciate the contribution and support in the sample visualisation provided by Dr. Kim Sewell and Dr. Matthias Floetenmeyer (CMM-UQ).
Open Access funding enabled and organized by CAUL and its Member Institutions
Author information
Authors and Affiliations
Contributions
Khanh Phan: Methodology, Investigation, Data curation, Writing - original draft, Writing - review & editing. Tuyen Truong: Investigation, Writing - review & editing, Supervision. Yong Wang: Investigation, Writing - review & editing, Supervision. Bhesh Bhandari: Conceptualization, Methodology, Writing - review & editing, Supervision.
Corresponding authors
Ethics declarations
Competing Interests
The authors declare no competing interests.
Declaration of Competing Interest
The authors declare that they have no conflicts of interest.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Electronic Supplementary Material
Below is the link to the electronic supplementary material.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article’s Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Phan, K., Truong, T., Wang, Y. et al. Generation and Influence of Carbon Dioxide Nanobubbles on Physicochemical Properties Including the Surface Tension of Clarified Apple Juice. Food Biophysics 19, 131–142 (2024). https://doi.org/10.1007/s11483-023-09810-w
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11483-023-09810-w