Abstract
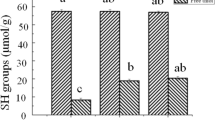
We investigated the effect of acrolein, a byproduct of lipid oxidation, on the structure and gel properties of myofibrillar proteins (MPs) isolated from rabbit meat. As the acrolein concentration increased, the protein carbonyl compounds significantly accumulated (p < 0.05), and the total sulfhydryl content was significantly lost (p < 0.05). The results of circular dichroism spectra, surface hydrophobicity, UV absorption spectra and intrinsic fluorescence spectra evidenced that acrolein caused the disruption of α-helix structure, the exposure of hydrophobic sites and the unfolding of MPs. Sodium dodecyl sulfate polyacrylamide gel electrophoresis (SDS-PAGE) analysis suggested that medium (0–1 mM) and high (5–10 mM) concentrations of acrolein could induce protein cross-linkage and protein aggregation, respectively. These structural changes could affect gelling properties of MPs involving gel strength and water holding capacity (WHC). The results of Raman spectroscopy indicated that moderate oxidative modification caused protein unfolding as well as the decline of α-helix structure and the increase of β-sheets structure in gels, thereby influencing the gel properties. Moderate oxidative modification (0–1 mM) improved gel strength and WHC, while excessive oxidative modification (5–10 mM) resulted in decreased gel properties.









Similar content being viewed by others
References
F. Zhou, M. Zhao, H. Zhao, W. Sun, C. Cui, Effects of oxidative modification on gel properties of isolated porcine myofibrillar protein by peroxyl radicals. Meat Sci. 96, 1432–1439 (2014)
X. Chen, R.K. Tume, Y. Xiong, X. Xu, G. Zhou, C. Chen, T. Nishiumi, Structural modification of myofibrillar proteins by high-pressure processing for functionally improved, value-added, and healthy muscle gelled foods. Crit. Rev. Food Sci. Nutr., 1–23 (2017)
W. Zhang, S. Xiao, D.U. Ahn, Protein oxidation: basic principles and implications for meat quality. Crit. Rev. Food Sci. Nutr. 53, 1191–1201 (2013)
M. Estévez, Protein carbonyls in meat systems: a review. Meat Sci. 89, 259–279 (2011)
S. Jongberg, M. N. Lund, L. H. Skibsted, Protein oxidation in meat and meat products. Challenges for antioxidative protection. In Global Food Security and Wellness, ed. by G. Barbosa-Cánovas, et al. (Springer, New York, 2017), pp. 315–337
L. Wang, M. Zhang, Z. Fang, B. Bhandari, Gelation properties of myofibrillar protein under malondialdehyde-induced oxidative stress. J. Sci. Food Agric. 97, 50–57 (2017)
M. Hęś, Protein-lipid interactions in different meat systems in the presence of natural antioxidants–a review. Polish J. Food Nutr. Sci. 67, 5–18 (2017)
V.M. Osório, Z. de Lourdes Cardeal, Determination of acrolein in french fries by solid-phase microextraction gas chromatography and mass spectrometry. J. Chromatogr. A 1218, 3332–3336 (2011)
B.C. Sousa, A.R. Pitt, C.M. Spickett, Chemistry and analysis of HNE and other prominent carbonyl-containing lipid oxidation compounds. Free Radic. Biol. Med. 111, 294–308 (2017)
S. Pizzimenti, E.S. Ciamporcero, M. Daga, P. Pettazzoni, A. Arcaro, G. Cetrangolo, Interaction of aldehydes derived from lipid peroxidation and membrane proteins. Front. Physiol. 4, 242–249 (2013)
V.R. Thompson, A.P. DeCaprio, Covalent adduction of nitrogen mustards to model protein nucleophiles. Chem. Res. Toxicol. 26, 1263–1271 (2013)
P. Hernández, A. Dalle Zotte, Influence of diet on rabbit meat quality. In Nutrition of the Rabbit, ed. by P. Hernández, A. Dalle Zotte (CABI, 2010), pp.163–178
I. Chelh, P. Gatellier, V. Santé-Lhoutellier, A simplified procedure for myofibril hydrophobicity determination. Meat Sci. 74, 681–683 (2006)
C. Qiu, W. Xia, Q. Jiang, Pressure-induced changes of silver carp (Hypophthalmichthys molitrix) myofibrillar protein structure. Eur. Food Res. Technol. 238(5), 753–761 (2014)
W. Jiang, Y. He, S. Xiong, Y. Liu, T. Yin, Y. Hu, J. You, Effect of mild ozone oxidation on structural changes of silver carp (Hypophthalmichthys molitrix) myosin. Food Bioprocess Technol. 10, 370–378 (2017)
L. Lv, H. Lin, Z. Li, J. Wang, I. Ahmed, H. Chen, Changes of structure and IgE binding capacity of shrimp (Metapenaeus ensis) tropomyosin followed by acrolein treatment. Food Funct. 8, 1028–1036 (2017)
X. Zhuang, M. Han, Y. Bai, Y. Liu, L. Xing, X.L. Xu, G.H. Zhou, Insight into the mechanism of myofibrillar protein gel improved by insoluble dietary fiber. Food Hydrocoll. 74, 219–226 (2018)
W. Wu, X. Wu, Y. Hua, Structural modification of soy protein by the lipid peroxidation product acrolein. LWT – Food Sci. Technol. 4, 133–140 (2010)
G. Aldini, M. Orioli, M. Carini, Protein modification by acrolein: relevance to pathological conditions and inhibition by aldehyde sequestering agents. Mol. Nutr. Food Res. 55, 1301–1319 (2011)
J. Cai, A. Bhatnagar, W.M. Pierce Jr., Protein modification by acrolein: formation and stability of cysteine adducts. Chem. Res. Toxicol. 22, 708–716 (2009)
T. Maeshima, K. Honda, M. Chikazawa, T. Shibata, Y. Kawai, M. Akagawa, et al., Quantitative analysis of acrolein-specific adducts generated during lipid peroxidation–modification of proteins in vitro: Identification of N τ-(3-propanal) histidine as the major adduct. Chem. Res. Toxicol. 25, 1384–1392 (2012)
K. Uchida, M. Kanematsu, K. Sakai, T. Matsuda, N. Hattori, Y. Mizuno, et al., Protein-bound acrolein: potential markers for oxidative stress. Proc. Natl. Acad. Sci. 95, 4882–4887 (1998)
A. Furuhata, T. Ishii, S. Kumazawa, T. Yamada, T. Nakayama, K. Uchida, Nϵ-(3-Methylpyridinium) lysine, a major antigenic adduct generated in acrolein-modified protein. J. Biol. Chem. 278, 48658–48665 (2003)
L.M. Kaminskas, S.M. Pyke, P.C. Burcham, Michael addition of acrolein to lysinyl and N-terminal residues of a model peptide: targets for cytoprotective hydrazino drugs. Rapid Commun. Mass Spectrom. 21, 1155–1164 (2007)
M.J. Randall, P.C. Spiess, M. Hristova, R.J. Hondal, A. van der Vliet, Acrolein-induced activation of mitogen-activated protein kinase signaling is mediated by alkylation of thioredoxin reductase and thioredoxin 1. Redox Biol. 1, 265–275 (2013)
A. Furuhata, M. Nakamura, T. Osawa, K. Uchida, Thiolation of protein-bound carcinogenic aldehyde an electrophilic acrolein-lysine adduct that covalently binds to thiols. J. Biol. Chem. 277, 27919–27926 (2002)
Z. Zhang, Y. Yang, P. Zhou, X. Zhang, J. Wang, Effects of high pressure modification on conformation and gelation properties of myofibrillar protein. Food Chem. 217, 678–686 (2017)
N. Jia, L. Wang, J. Shao, D. Liu, B. Kong, Changes in the structural and gel properties of pork myofibrillar protein induced by catechin modification. Meat Sci. 127, 45–50 (2017)
Z.J. Bao, J.P. Wu, Y. Cheng, Y.J. Chi, Effects of lipid peroxide on the structure and gel properties of ovalbumin. Process Biochem. 57, 124–130 (2017)
L. Lv, H. Lin, Z. Li, F. Yuan, Q. Gao, J. Ma, Effect of 4-hydroxy-2-nonenal treatment on the IgE binding capacity and structure of shrimp (Metapenaeus ensis) tropomyosin. Food Chem. 212, 313–322 (2016)
Z. Wang, Z. He, H. Li, Mass transfer dynamics during brining of rabbit meat. World Rabbit Sci. 25, 377–385 (2017)
L. Wang, M. Zhang, Z. Fang, B. Bhandari, Z. Gao, Influence of linoleic acid-induced oxidative modification on gel properties of myofibrillar protein from silver carp (Hypophthalmichthys molitrix) muscle. Food Biophys. 11, 266–274 (2016)
K.Q. Wang, S.Z. Luo, X.Y. Zhong, J. Cai, S.T. Jiang, Z. Zheng, Changes in chemical interactions and protein conformation during heat-induced wheat gluten gel formation. Food Chem. 214, 393–399 (2017)
M. Zhang, F. Li, X. Diao, B. Kong, X. Xia, Moisture migration, microstructure damage and protein structure changes in porcine longissimus muscle as influenced by multiple freeze-thaw cycles. Meat Sci. 133, 10–18 (2017)
A. Segat, N.N. Misra, A. Fabbro, F. Buchini, G. Lippe, P.J. Cullen, N. Innocente, Effects of ozone processing on chemical, structural and functional properties of whey protein isolate. Food Res. Int. 66, 365–372 (2014)
S.J. Li, A.J. King, Structural changes of rabbit myosin subfragment 1 altered by malonaldehyde, a byproduct of lipid oxidation. J. Agric. Food Chem. 47, 3124–3129 (1999)
T. Ishii, T. Yamada, T. Mori, S. Kumazawa, K. Uchida, T. Nakayama, Characterization of acrolein-induced protein cross-links. Free Radic. Res. 41, 1253–1260 (2007)
Y. Sun, H. Luo, J. Cao, D. Pan, Structural characteristics of Sheldrake meat and secondary structure of myofibrillar protein: effects of oxidation. Int. J. Food Prop. 20, 1553–1566 (2017)
Y.L. Xiong, S.P. Blanchard, T. Ooizumi, Y. Ma, Hydroxyl radical and Ferryl-generating systems promote gel network formation of myofibrillar protein. J. Food Sci. 75, 215–221 (2010)
F. Zhou, M. Zhao, G. Su, C. Cui, W. Sun, Gelation of salted myofibrillar protein under malondialdehyde-induced oxidative stress. Food Hydrocoll. 40, 153–162 (2014)
M. Wang, X. Chen, Y. Zou, H. Chen, S. Xue, C. Qian, P. Wang, X. Xu, G. Zhou, High-pressure processing-induced conformational changes during heating affect water holding capacity of myosin gel. Int. J. Food Sci Technol. 52, 724–732 (2017)
L. Wang, M. Zhang, B. Bhandari, Z. Gao, Effects of malondialdehyde-induced protein modification on water functionality and physicochemical state of fish myofibrillar protein gel. Food Res. Int. 86, 131–139 (2016)
H. Yang, W. Zhang, T. Li, H. Zheng, M.A. Khan, X. Xu, et al., Effect of protein structure on water and fat distribution during meat gelling. Food Chem. 204, 239–245 (2016)
C.B. Tang, W.G. Zhang, Y.F. Zou, L.J. Xing, H.B. Zheng, X.L. Xu, G.H. Zhou, Influence of RosA-protein adducts formation on myofibrillar protein gelation properties under oxidative stress. Food Hydrocoll. 67, 197–205 (2017)
S. Xue, X. Yu, H. Yang, X. Xu, H. Ma, G. Zhou, Contribution of high-pressure-induced protein modifications to the microenvironment and functional properties of rabbit meat sausages. J. Food Sci. 82(6), 1357–1368 (2017)
Acknowledgements
The authors gratefully acknowledge financial support from General Program of National Natural Science Foundation of China (31671787), the National Rabbit Industry Technology System Programme (Grant No. CARS-43-E-1) and Chongqing Herbivorous livestock Industry Technology System (Y201706).
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of Interest
The authors declare that we have no conflict of interest.
Rights and permissions
About this article
Cite this article
Wang, Z., He, Z., Gan, X. et al. The Effects of Lipid Oxidation Product Acrolein on the Structure and Gel Properties of Rabbit Meat Myofibrillar Proteins. Food Biophysics 13, 374–386 (2018). https://doi.org/10.1007/s11483-018-9543-6
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11483-018-9543-6