Abstract
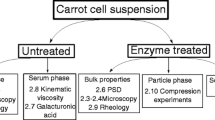
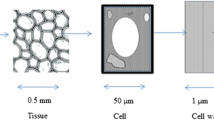
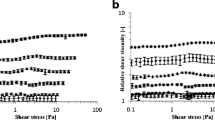
The relationship between small amplitude oscillatory rheological properties and microstructure of plant cell suspensions was studied. Carrot, broccoli and tomato were selected as model plant systems to generate particles with different microstructures: clusters of cells with smooth or rough edges and single cells. By analysing the compressive stress undergone by the plant cells under centrifugation, and comparing this to oscillatory rheometry, agreement was found between the compressive stress required to compress the dispersions to higher insoluble solids dry mass fractions, and the elastic shear modulus of the plant dispersions. This indicated that centrifugation is acting as a crude rheological measurement on the samples, rather than measuring any well-defined “particle phase volume”. We estimated the theoretical critical dry mass fraction above which smooth, roughly spherical, elastically interacting particles would acquire a non-zero G′, and compared this with the experimental values. Our results give evidence that for the three vegetable suspensions considered here, the elastic rheology observed is not coming simply from the packing of smooth particles, but is dominated in the dilute limit by attractive forces or interaction of asperities, and in the concentrated limit by deformation and buckling acting together. Improved understanding of the particles and their packing would help in the structuring of food products without adding other texturising or stabilising agents.







Similar content being viewed by others
References
C.W. Macosko, Rheology: Principles, Measurements and Applications (VCH publishers, New York, 1994), pp. 425–474
D.B. Genovese, J.E. Lozano, M.A. Rao, The rheology of colloidal and non-colloidal food dispersions. J. Food Sci. 72, R11–R20 (2007)
L. Day, M. Xu, S.K. Øiseth, L. Lundin, Y. Hemar, Dynamic rheological properties of plant cell-wall particle dispersions. Colloids Surf. B Biointerfaces 81, 461–467 (2010)
P. Lopez-Sanchez, J. Nijjsse, H.C.G. Blonk, L. Bialek, S. Schumm, M. Langton, Effect of mechanical and thermal treatments on the microstructure and the rheological properties of carrot, broccoli and tomato dispersions. J. Sci. Food Agric. 91, 207–217 (2011)
I. Shomer, P. Lindner, R. Vasiliver, Mechanism which enables the cell wall to retain homogeneous appearance of tomato juice. J. Food Sci. 49, 628–633 (1984)
R.S. Farr, R.D. Groot, Close packing density of polidisperse hard spheres. J. Chem. Phys. 131, 244104 (2009)
Q.D. Nguyen, D.V. Boger, Yield stress measurement for concentrated suspensions. J. Rheol. 27, 321–349 (1983)
L.C. Greve, R.N. McArdle, J.R. Gohlke, J.M. Labavitch, Impact of heating on carrot firmness: changes in cell wall components. J. Agric. Food Chem. 42, 2900–2906 (1994)
T.G. Mason, J. Bibette, D.A. Weitz, Elasticity of compressed emulsions. Phys. Rev. Lett. 75, 2051–2054 (1995)
T.G. Mason, J. Bibette, D.A. Weitz, Yielding and flow of monodispersed emulsions. J. Colloid Interface Sci. 179, 439–448 (1996)
J.D. Bernal, J. Mason, Packing of spheres: co-ordination of randomly packed spheres. Nature 188, 910–911 (1960)
D.A. Weitz, M. Oliveria, Fractal structures formed by kinetic aggregation of aqueous gold colloids. Phys. Rev. Lett. 52, 1433–1436 (1984)
C. Song, P. Wang, H.A. Makse, A phase diagram for jammed matter. Nature 453, 629–632 (2008)
G.W. Delaney, P.W. Cleary, The packing properties of superellipsoids. Europhys. Lett. 89, 34002 (2010)
Acknowledgments
The authors would like to thank Maud Langton for proof reading of manuscript draft. This work was financially supported by the Commission of the European Communities, Framework 6, Priority 5 ‘Food Quality and Safety’, STREP Project Healthy Structuring 2006–023115.
Author information
Authors and Affiliations
Corresponding author
Appendix
Appendix
In the approximate analysis in the body of the text, the volume fraction of insoluble solids in the sediment, ϕ(z), was assumed to be independent of z. However, in reality, the sediment column will not be uniform, since particles further from the surface will be compressed by a greater weight of overlying particles. To proceed, we make the assumption that there is a power law relationship between the local osmotic pressure and the local volume fraction of insoluble solids, viz:.
Where Π0 and n are constants, and the validity of this power law can be checked a posteriori.
Then from Eq. 10 in the text,
so
where C is an integration constant, which vanishes because the osmotic pressure at the top of the sediment column is zero. Thus
Now, the volume of insoluble solids before centrifugation is
where A is the cross sectional area of the column, and after centrifugation
Therefore
From Eqs. A.1 and A.2 ϕ in terms of z will be:
And so, simplifying slightly, we obtain:
which can be re-arranged to give
What is known experimentally, is \( \Psi \equiv \frac{h}{{{h_0}}} \) in terms of ϕ 0, and we also know the values of h0, as well as ρc and ρserum [the latter from Eq. 6 in the text].
We can thus plot h against ϕ 0 from the centrifugation data, and fit this to Eq. A.5 to obtain the best fitting values for n and Π0. Once we have those values, Eq. A.1 is used to plot Π vs ϕ 0.
- Π:
-
osmotic pressure from compressive stress (Pa)
- ρ c :
-
density of dry carbohydrates (kg m−3)
- ρ w :
-
density of water (kg m−3)
- ρ serum :
-
density of serum phase in each sample (kg m−3)
- ϕ 0 :
-
volume fraction of insoluble solids in sample before centrifugation (no units)
- ϕ :
-
volume fraction of insoluble solids in a local region of a sample (no units)
- ϕ sed :
-
volume fraction of insoluble solids in the sediment (no units)
- ϕ*:
-
critical volume fraction of insoluble solids at particle close packing (no units)
- Ψ:
-
apparent volume fraction (ratio of sediment height to sample height) (no units)
- Ω:
-
mass ratio Msed/M (no units)
- A:
-
surface area of one cell (m2)
- D:
-
diameter of one cell (m)
- h0 :
-
height of centrifugation sample (m)
- h:
-
height of sediment after centrifugation (m)
- M:
-
mass of centrifugation sample (kg)
- Msed :
-
wet mass sediment after centrifugation (kg)
- t:
-
thickness of the cell wall (m)
- w:
-
dry mass fraction of sample (no units)
- \( w_{{total}}^{{veg}} \) :
-
dry mass fraction of all solids in vegetable of type ‘veg’ (no units)
- \( w_{{ins}}^{{veg}} \) :
-
dry mass fraction of insoluble solids in vegetable of type ‘veg’ (no units)
- \( w_{{sol}}^{{veg}} \) :
-
dry mass fraction of soluble solids in vegetable of type ‘veg’ (no units)
- \( w_{{serum}}^{{veg}} \) :
-
dry mass fraction of soluble solids in the serum from vegetable type ‘veg’ (no units)
- \( \hat{w}_{{wet}}^{{veg}} \) :
-
wet mass fraction of vegetable type ‘veg’ in the centrifugation sample (no units)
- wins :
-
dry mass fraction of insoluble solids in sample (no units)
- wsol :
-
dry mass fraction of soluble solids (assumed carbohydrates) in sample (no units)
Rights and permissions
About this article
Cite this article
Lopez-Sanchez, P., Chapara, V., Schumm, S. et al. Shear Elastic Deformation and Particle Packing in Plant Cell Dispersions. Food Biophysics 7, 1–14 (2012). https://doi.org/10.1007/s11483-011-9237-9
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11483-011-9237-9