Abstract
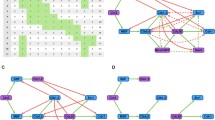
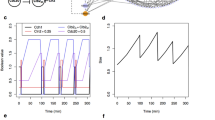
From the first application of the Boolean model to the cell cycle regulation network of budding yeast, new regulative pathways have been discovered, particularly in the G1/S transition circuit. This discovery called for finer modeling to study the essential biology, and the resulting outcomes are first introduced in the article. A traditional Boolean network model set up for the new G1/S transition circuit shows that it cannot correctly simulate real biology unless the model parameters are fine tuned. The deficiency is caused by an overly coarsegrained description of the inhibitor binding process, which shall be overcome by a two-vector model proposed whose robustness is surveyed using random perturbations. Simulations show that the proposed two-vector model is much more robust in describing inhibitor binding processes within the Boolean framework.
Similar content being viewed by others
References
Bornholdt S. Systems biology: Less is more in modeling large genetic networks. Science, 2005, 310(5747): 449–451
Li F T, Long T, Lu Y, et al. The yeast cell-cycle network is robustly designed. Proceedings of the National Academy of Sciences of the United States of America, 2004, 101(14): 4781–4786
Kauffman S A. The Origins of Order: Self-Organization and Selection in Evolution. NewYork: Oxford University Press, 1993
Albert R, Othmer H G. The topology of the regulatory interactions predicts the expression pattern of the segment polarity genes in Drosophila melanogaster. Journal of Theoretical Biology, 2003, 223(1): 1–18
Davidich M I, Bornholdt S. Boolean network model predicts cell cycle sequence of fission yeast. PLoS ONE, 2008, 3(2): e1672
Rubinstein A, Gurevich V, Kasulin-Boneh, et al. Faithful modeling of transient expression and its application to elucidating negative feedback regulation. Proceedings of the National Academy of Sciences of the United States of America, 2007 104(15): 6241–6246
de Bruin R A, Kalashnikova T I, Chahwan C, et al. Constraining G1-specific transcription to late G1 phase: the MBF-associated corepressor Nrm1 acts via negative feedback. Molecular Cell, 2006, 23(4): 483–496
de Bruin R A, McDonald W H, Kalashnikova T I, et al. Cln3 activates G1-specific transcription via phosphorylation of the SBF bound repressor Whi5. Cell, 2004, 117(7): 887–898
Costanzo M, Nishikawa J L, Tang X, et al. CDK activity antagonizes Whi5, an inhibitor of G1/S transcription in yeast. Cell, 2004, 117(7): 899–913
Braunewell S, Bornholdt S. Superstability of the yeast cell-cycle dynamics: ensuring causality in the presence of biochemical stochasticity. Journal of Theoretical Biology, 2007, 245(4): 638–643
Morgan D O. The Cell Cycle: Principles of Control. New York: Oxford University Press, 2006, Chapter 3
Verma R, Annan R S, Huddleston M J, et al. Phosphorylation of Sic1p by G1 Cdk required for its degradation and entry into S phase. Science, 1997, 278(5337): 455–460
Author information
Authors and Affiliations
Corresponding author
About this article
Cite this article
Xie, Z.S., Tang, C. A more robust Boolean model describing inhibitor binding. Front. Electr. Electron. Eng. China 3, 371–375 (2008). https://doi.org/10.1007/s11460-008-0079-2
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11460-008-0079-2