Abstract
Transgenic models are useful tools for studying the pathogenesis of and drug development for Alzheimer’s Disease (AD). AD models are constructed usually using overexpression or knock-in of multiple pathogenic gene mutations from familial AD. Each transgenic model has its unique behavioral and pathological features. This review summarizes the research progress of transgenic mouse models, and their progress in the unique mechanism of amyloid-β oligomers, including the first transgenic mouse model built in China based on a single gene mutation (PSEN1 V97L) found in Chinese familial AD. We further summarized the preclinical findings of drugs using the models, and their future application in exploring the upstream mechanisms and multitarget drug development in AD.
Similar content being viewed by others
References
Adalbert, R., Nogradi, A., Babetto, E., Janeckova, L., Walker, S.A., Kerschensteiner, M., Misgeld, T., and Coleman, M.P. (2009). Severely dystrophic axons at amyloid plaques remain continuous and connected to viable cell bodies. Brain 132, 402–416.
Alexander, A.G., Marfil, V., and Li, C. (2014). Use of Caenorhabditis elegans as a model to study Alzheimer’s disease and other neurodegenerative diseases. Front Genet 5, 279.
Almkvist, O., Rodriguez-Vieitez, E., Thordardottir, S., Nordberg, A., Viitanen, M., Lannfelt, L., and Graff, C. (2019). Longitudinal cognitive decline in autosomal-dominant Alzheimer’s disease varies with mutations in APP and PSEN1 genes. Neurobiol Aging 82, 40–47.
An, J., Zhou, Y., Zhang, M., Xie, Y., Ke, S., Liu, L., Pan, X., and Chen, Z. (2019). Exenatide alleviates mitochondrial dysfunction and cognitive impairment in the 5×FAD mouse model of Alzheimer’s disease. Behav Brain Res 370, 111932.
Ano, Y., Takaichi, Y., Ohya, R., Uchida, K., Nakayama, H., and Takashima, A. (2023). Tryptophan-tyrosine dipeptide improves tau-related symptoms in tauopathy mice. Nutr Neurosci 26, 766–777.
Arancio, O., Zhang, H.P., Chen, X., Lin, C., Trinchese, F., Puzzo, D., Liu, S., Hegde, A., Yan, S.F., Stern, A., et al. (2004). RAGE potentiates Aβ-induced perturbation of neuronal function in transgenic mice. EMBO J 23, 4096–4105.
Arendash, G.W., King, D.L., Gordon, M.N., Morgan, D., Hatcher, J.M., Hope, C.E., and Diamond, D.M. (2001). Progressive, age-related behavioral impairments in transgenic mice carrying both mutant amyloid precursor protein and presenilin-1 transgenes. Brain Res 891, 42–53.
Armbrust, F., Bickenbach, K., Marengo, L., Pietrzik, C., and Becker-Pauly, C. (2022). The Swedish dilemma—the almost exclusive use of APPswe-based mouse models impedes adequate evaluation of alternative β-secretases. Biochim Biophys Acta 1869, 119164.
Baglietto-Vargas, D., Forner, S., Cai, L., Martini, A.C., Trujillo-Estrada, L., Swarup, V., Nguyen, M.M.T., Do Huynh, K., Javonillo, D.I., Tran, K. M., et al. (2021). Generation of a humanized Aβ expressing mouse demonstrating aspects of Alzheimer’s disease-like pathology. Nat Commun 12, 2421.
Barrow, P., Villabruna, L., Hoberman, A., Bohrmann, B., Richter, W.F., and Schubert, C. (2017). Reproductive and developmental toxicology studies with gantenerumab in PS2APP transgenic mice. Reprod Toxicol 73, 362–371.
Barrow, P.A., Empson, R.M., Gladwell, S.J., Anderson, C.M., Killick, R., Yu, X., Jefferys, J.G.R., and Duff, K. (2000). Functional phenotype in transgenic mice expressing mutant human presenilin-1. Neurobiol Dis 7, 119–126.
Bayer, T.A. (2022). Pyroglutamate Aβ cascade as drug target in Alzheimer’s disease. Mol Psychiatry 27, 1880–1885.
Begley, J.G., Duan, W., Chan, S., Duff, K., and Mattson, M.P. (1999). Altered calcium homeostasis and mitochondrial dysfunction in cortical synaptic compartments of presenilin-1 mutant mice. J Neurochem 72, 1030–1039.
Bellucci, A., Rosi, M.C., Grossi, C., Fiorentini, A., Luccarini, I., and Casamenti, F. (2007). Abnormal processing of tau in the brain of aged TgCRND8 mice. Neurobiol Dis 27, 328–338.
Bellucci, A., Westwood, A.J., Ingram, E., Casamenti, F., Goedert, M., and Spillantini, M.G. (2004). Induction of inflammatory mediators and microglial activation in mice transgenic for mutant human P301S tau protein. Am J Pathol 165, 1643–1652.
Billings, L.M., Oddo, S., Green, K.N., McGaugh, J.L., and LaFerla, F.M. (2005). Intraneuronal Aβ causes the onset of early Alzheimer’s disease-related cognitive deficits in transgenic mice. Neuron 45, 675–688.
Bittner, T., Burgold, S., Dorostkar, M.M., Fuhrmann, M., Wegenast-Braun, B.M., Schmidt, B., Kretzschmar, H., and Herms, J. (2012). Amyloid plaque formation precedes dendritic spine loss. Acta Neuropathol 124, 797–807.
Blázquez, G., Cañete, T., Tobeña, A., Giménez-Llort, L., and Fernández-Teruel, A. (2014). Cognitive and emotional profiles of aged Alzheimer’s disease (3×TgAD) mice: Effects of environmental enrichment and sexual dimorphism. Behav Brain Res 268, 185–201.
Boekhoorn, K., Terwel, D., Biemans, B., Borghgraef, P., Wiegert, O., Ramakers, G.J.A., de Vos, K., Krugers, H., Tomiyama, T., Mori, H., et al. (2006). Improved long-term potentiation and memory in young tau-P301L transgenic mice before onset of hyperphosphorylation and tauopathy. J Neurosci 26, 3514–3523.
Boncristiano, S., Calhoun, M.E., Howard, V., Bondolfi, L., Kaeser, S.A., Wiederhold, K.H., Staufenbiel, M., and Jucker, M. (2005). Neocortical synaptic bouton number is maintained despite robust amyloid deposition in APP23 transgenic mice. Neurobiol Aging 26, 607–613.
Borchelt, D.R., Ratovitski, T., van Lare, J., Lee, M.K., Gonzales, V., Jenkins, N.A., Copeland, N.G., Price, D.L., and Sisodia, S.S. (1997). Accelerated amyloid deposition in the brains of transgenic mice coexpressing mutant presenilin 1 and amyloid precursor proteins. Neuron 19, 939–945.
Borchelt, D.R., Thinakaran, G., Eckman, C.B., Lee, M.K., Davenport, F., Ratovitsky, T., Prada, C.M., Kim, G., Seekins, S., Yager, D., et al. (1996). Familial Alzheimer’s disease-linked presenilin 1 variants elevate Aβ1–42/1–40 ratio in vitro and in vivo. Neuron 17, 1005–1013.
Bouter, Y., Dietrich, K., Wittnam, J.L., Rezaei-Ghaleh, N., Pillot, T., Papot-Couturier, S., Lefebvre, T., Sprenger, F., Wirths, O., Zweckstetter, M., et al. (2013). N-truncated amyloid β (Aβ) 4–42 forms stable aggregates and induces acute and long-lasting behavioral deficits. Acta Neuropathol 126, 189–205.
Brautigam, H., Steele, J.W., Westaway, D., Fraser, P.E., George-Hyslop, P. H.S., Gandy, S., Hof, P.R., and Dickstein, D.L. (2012). The isotropic fractionator provides evidence for differential loss of hippocampal neurons in two mouse models of Alzheimer’s disease. Mol Neurodegener 7, 58.
Breyhan, H., Wirths, O., Duan, K., Marcello, A., Rettig, J., and Bayer, T.A. (2009). APP/PS1KI bigenic mice develop early synaptic deficits and hippocampus atrophy. Acta Neuropathol 117, 677–685.
Brown, J.T., Richardson, J.C., Collingridge, G.L., Randall, A.D., and Davies, C.H. (2005). Synaptic transmission and synchronous activity is disrupted in hippocampal slices taken from aged TAS10 mice. Hippocampus 15, 110–117.
Bruggink, K.A., Jongbloed, W., Biemans, E.A.L.M., Veerhuis, R., Claassen, J.A.H.R., Kuiperij, H.B., and Verbeek, M.M. (2013). Amyloid-β oligomer detection by ELISA in cerebrospinal fluid and brain tissue. Anal Biochem 433, 112–120.
Buskila, Y., Crowe, S.E., and Ellis-Davies, G.C.R. (2013). Synaptic deficits in layer 5 neurons precede overt structural decay in 5xFAD mice. Neuroscience 254, 152–159.
Cacciottolo, M., Morgan, T.E., and Finch, C.E. (2021). Age, sex, and cerebral microbleeds in EFAD Alzheimer disease mice. Neurobiol Aging 103, 42–51.
Calhoun, M.E., Wiederhold, K.H., Abramowski, D., Phinney, A.L., Probst, A., Sturchler-Pierrat, C., Staufenbiel, M., Sommer, B., and Jucker, M. (1998). Neuron loss in APP transgenic mice. Nature 395, 755–756.
Capitanio, J.P., and Emborg, M.E. (2008). Contributions of non-human primates to neuroscience research. Lancet 371, 1126–1135.
Caruso, D., Barron, A.M., Brown, M.A., Abbiati, F., Carrero, P., Pike, C.J., Garcia-Segura, L.M., and Melcangi, R.C. (2013). Age-related changes in neuroactive steroid levels in 3xTg-AD mice. Neurobiol Aging 34, 1080–1089.
Casas, C., Sergeant, N., Itier, J.M., Blanchard, V., Wirths, O., van der Kolk, N., Vingtdeux, V., van de Steeg, E., Ret, G., Canton, T., et al. (2004). Massive CA1/2 neuronal loss with intraneuronal and N-terminal truncated Aβ42 accumulation in a novel Alzheimer transgenic model. Am J Pathol 165, 1289–1300.
Chang, L., Bakhos, L., Wang, Z., Venton, D.L., and Klein, W.L. (2003). Femtomole immunodetection of synthetic and endogenous amyloid-β oligomers and its application to Alzheimer’s Disease drug candidate screening. J Mol Neurosci 20, 305–314.
Chapman, P.F., White, G.L., Jones, M.W., Cooper-Blacketer, D., Marshall, V.J., Irizarry, M., Younkin, L., Good, M.A., Bliss, T.V.P., Hyman, B.T., et al. (1999). Impaired synaptic plasticity and learning in aged amyloid precursor protein transgenic mice. Nat Neurosci 2, 271–276.
Chen, S., and Jia, J. (2020). Tenuifolin attenuates amyloid-β42-induced neuroinflammation in microglia through the NF-κB signaling pathway. J Alzheimer Dis 76, 195–205.
Chen, S.Y., Gao, Y., Sun, J.Y., Meng, X.L., Yang, D., Fan, L.H., Xiang, L., and Wang, P. (2020). Traditional Chinese medicine: role in reducing β-amyloid, apoptosis, autophagy, neuroinflammation, oxidative stress, and mitochondrial dysfunction of Alzheimer’s disease. Front Pharmacol 11, 497.
Chen, Y., Wang, Y., Qin, Q., Zhang, Y., Xie, L., Xiao, J., Cao, Y., Su, Z., and Chen, Y. (2022). Carnosic acid ameliorated Aβ-mediated (amyloid-β peptide) toxicity, cholinergic dysfunction and mitochondrial defect in Caenorhabditis elegans of Alzheimer’s Model. Food Funct 13, 4624–4640.
Cheng-Hathaway, P.J., Reed-Geaghan, E.G., Jay, T.R., Casali, B.T., Bemiller, S.M., Puntambekar, S.S., von Saucken, V.E., Williams, R. Y., Karlo, J.C., Moutinho, M., et al. (2018). The Trem2 R47H variant confers loss-of-function-like phenotypes in Alzheimer’s disease. Mol Neurodegener 13, 29.
Cheng, I.H., Palop, J.J., Esposito, L.A., Bien-Ly, N., Yan, F., and Mucke, L. (2004). Aggressive amyloidosis in mice expressing human amyloid peptides with the Arctic mutation. Nat Med 10, 1190–1192.
Cheng, I.H., Scearce-Levie, K., Legleiter, J., Palop, J.J., Gerstein, H., Bien-Ly, N., Puolivaöli, J., Lesné, S., Ashe, K.H., Muchowski, P.J., et al. (2007). Accelerating amyloid-β fibrillization reduces oligomer levels and functional deficits in Alzheimer disease mouse models. J Biol Chem 282, 23818–23828.
Cheong, S.L., Tiew, J.K., Fong, Y.H., Leong, H.W., Chan, Y.M., Chan, Z. L., and Kong, E.W.J. (2022). Current pharmacotherapy and multi-target approaches for Alzheimer’s disease. Pharmaceuticals 15, 1560.
Chishti, M.A., Yang, D.S., Janus, C., Phinney, A.L., Horne, P., Pearson, J., Strome, R., Zuker, N., Loukides, J., French, J., et al. (2001). Early-onset amyloid deposition and cognitive deficits in transgenic mice expressing a double mutant form of amyloid precursor protein 695. J Biol Chem 276, 21562–21570.
Cocco, S., Rinaudo, M., Fusco, S., Longo, V., Gironi, K., Renna, P., Aceto, G., Mastrodonato, A., Li Puma, D.D., Podda, M.V., et al. (2020). Plasma BDNF levels following transcranial direct current stimulation allow prediction of synaptic plasticity and memory deficits in 3×Tg-AD mice. Front Cell Dev Biol 8, 541.
Codita, A., Gumucio, A., Lannfelt, L., Gellerfors, P., Winblad, B., Mohammed, A.H., and Nilsson, L.N.G. (2010). Impaired behavior of female tg-ArcSwe APP mice in the IntelliCage: A longitudinal study. Behav Brain Res 215, 83–94.
Cohen, R.M., Rezai-Zadeh, K., Weitz, T.M., Rentsendorj, A., Gate, D., Spivak, I., Bholat, Y., Vasilevko, V., Glabe, C.G., Breunig, J.J., et al. (2013). A transgenic Alzheimer rat with plaques, tau pathology, behavioral impairment, oligomeric Aβ, and frank neuronal loss. J Neurosci 33, 6245–6256.
Colton, C.A., Wilcock, D.M., Wink, D.A., Davis, J., Van Nostrand, W.E., and Vitek, M.P. (2008). The effects of NOS2 gene deletion on mice expressing mutated human AbetaPP. J Alzheimers Dis 15, 571–587.
Congdon, E.E., Pan, R., Jiang, Y., Sandusky-Beltran, L.A., Dodge, A., Lin, Y., Liu, M., Kuo, M.H., Kong, X.P., and Sigurdsson, E.M. (2022). Single domain antibodies targeting pathological tau protein: Influence of four IgG subclasses on efficacy and toxicity. Ebiomedicine 84, 104249.
Cook, C., Dunmore, J.H., Murray, M.E., Scheffel, K., Shukoor, N., Tong, J., Castanedes-Casey, M., Phillips, V., Rousseau, L., Penuliar, M.S., et al. (2014). Severe amygdala dysfunction in a MAPT transgenic mouse model of frontotemporal dementia. Neurobiol Aging 35, 1769–1777.
Coppola, G., Chinnathambi, S., Lee, J.J.Y., Dombroski, B.A., Baker, M.C., Soto-Ortolaza, A.I., Lee, S.E., Klein, E., Huang, A.Y., Sears, R., et al. (2012). Evidence for a role of the rare p.A152T variant in MAPT in increasing the risk for FTD-spectrum and Alzheimer’s diseases. Hum Mol Genet 21, 3500–3512.
Cramer, P.E., Cirrito, J.R., Wesson, D.W., Lee, C.Y.D., Karlo, J.C., Zinn, A. E., Casali, B.T., Restivo, J.L., Goebel, W.D., James, M.J., et al. (2012). ApoE-directed therapeutics rapidly clear β-amyloid and reverse deficits in AD mouse models. Science 335, 1503–1506.
Cross, D.J., Huber, B.R., Silverman, M.A., Cline, M.M., Gill, T.B., Cross, C.G., Cook, D.G., and Minoshima, S. (2021). Intranasal paclitaxel alters Alzheimer’s disease phenotypic features in 3xTg-AD mice. J Alzheimer Dis 83, 379–394.
Crous-Bou, M., Minguillón, C., Gramunt, N., and Molinuevo, J.L. (2017). Alzheimer’s disease prevention: from risk factors to early intervention. Alzheimers Res Ther 9, 71.
Crouzin, N., Baranger, K., Cavalier, M., Marchalant, Y., Cohen-Solal, C., Roman, F.S., Khrestchatisky, M., Rivera, S., Feron, F., and Vignes, M. (2013). Area-specific alterations of synaptic plasticity in the 5XFAD mouse model of Alzheimer’s disease: dissociation between somato-sensory cortex and hippocampus. PLoS ONE 8, e74667.
Cummings, J., Lee, G., Nahed, P., Kambar, M.E.Z.N., Zhong, K., Fonseca, J., and Taghva, K. (2022). Alzheimer’s disease drug development pipeline: 2022. Alzheimers Dement 8, e12295.
Davis, J., Xu, F., Deane, R., Romanov, G., Previti, M.L., Zeigler, K., Zlokovic, B.V., and Van Nostrand, W.E. (2004). Early-onset and robust cerebral microvascular accumulation of amyloid β-protein in transgenic mice expressing low levels of a vasculotropic Dutch/Iowa mutant form of amyloid β-protein precursor. J Biol Chem 279, 20296–20306.
Davis, J., Xu, F., Miao, J., Previti, M.L., Romanov, G., Ziegler, K., and Van Nostrand, W.E. (2006). Deficient cerebral clearance of vasculotropic mutant Dutch/Iowa double Aβ in human AβPP transgenic mice. Neurobiol Aging 27, 946–954.
de Oliveira, P., Cella, C., Locker, N., Ravindran, K.K.G., Mendis, A., Wafford, K., Gilmour, G., Dijk, D.J., and Winsky-Sommerer, R. (2022). Improved sleep, memory, and cellular pathological features of tauopathy, including the NLRP3 inflammasome, after chronic administration of trazodone in rTg4510 mice. J Neurosci 42, 3494–3509.
Decker, J.M., Krüger, L., Sydow, A., Dennissen, F.J., Siskova, Z., Mandelkow, E., and Mandelkow, E. (2016). The Tau/A152T mutation, a risk factor for frontotemporal-spectrum disorders, leads to NR2B receptor-mediated excitotoxicity. EMBO Rep 17, 552–569.
Decker, J.M., Krüger, L., Sydow, A., Zhao, S., Frotscher, M., Mandelkow, E., and Mandelkow, E.M. (2015). Pro-aggregant Tau impairs mossy fiber plasticity due to structural changes and Ca++ dysregulation. Acta Neuropathol Commun 3, 23.
Delrieu, J., Bateman, R.J., Touchon, J., Sabbagh, M., and Cummings, J. (2022). The future of AD clinical trials with the advent of anti-amyloid therapies: an CTAD Task force report. J Prev Alzheimers Dis 9, 393–399.
Dennissen, F.J.A., Anglada-Huguet, M., Sydow, A., Mandelkow, E., and Mandelkow, E.M. (2016). Adenosine A1 receptor antagonist rolofylline alleviates axonopathy caused by human Tau ΔK280. Proc Natl Acad Sci USA 113, 11597–11602.
Dewachter, I., Van Dorpe, J., Smeijers, L., Gilis, M., Kuipéri, C., Laenen, I., Caluwaerts, N., Moechars, D., Checler, F., Vanderstichele, H., et al. (2000). Aging increased amyloid peptide and caused amyloid plaques in brain of old APP/V717I transgenic mice by a different mechanism than mutant presenilin1. J Neurosci 20, 6452–6458.
Dodart, J.C., Meziane, H., Mathis, C., Bales, K.R., Paul, S.M., and Ungerer, A. (1999). Behavioral disturbances in transgenic mice overexpressing the V717F B-amyloid precursor protein. Behav Neurosci 113, 982–990.
Dodiya, H.B., Frith, M., Sidebottom, A., Cao, Y., Koval, J., Chang, E., and Sisodia, S.S. (2020). Synergistic depletion of gut microbial consortia, but not individual antibiotics, reduces amyloidosis in APPPS1-21 Alzheimer’s transgenic mice. Sci Rep 10, 8183.
Dodiya, H.B., Lutz, H.L., Weigle, I.Q., Patel, P., Michalkiewicz, J., Roman-Santiago, C.J., Zhang, C.M., Liang, Y., Srinath, A., Zhang, X., et al. (2022). Gut microbiota-driven brain Aβ amyloidosis in mice requires microglia. J Exp Med 219.
Domnitz, S.B., Robbins, E.M., Hoang, A.W., Garcia-Alloza, M., Hyman, B.T., Rebeck, G.W., Greenberg, S.M., Bacskai, B.J., and Frosch, M.P. (2005). Progression of cerebral amyloid angiopathy in transgenic mouse models of Alzheimer disease. J Neuropathol Exp Neurol 64, 588–594.
Drummond, E., and Wisniewski, T. (2017). Alzheimer’s disease: experimental models and reality. Acta Neuropathol 133, 155–175.
Dudal, S., Krzywkowski, P., Paquette, J., Morissette, C., Lacombe, D., Tremblay, P., and Gervais, F. (2004). Inflammation occurs early during the Aβ deposition process in TgCRND8 mice. Neurobiol Aging 25, 861–871.
Duff, K., Eckman, C., Zehr, C., Yu, X., Prada, C.M., Perez-Tur, J., Hutton, M., Buee, L., Harigaya, Y., Yager, D., et al. (1996). Increased amyloid-β42(43) in brains of mice expressing mutant presenilin 1. Nature 383, 710–713.
Eckermann, K., Mocanu, M.M., Khlistunova, I., Biernat, J., Nissen, A., Hofmann, A., Schönig, K., Bujard, H., Haemisch, A., Mandelkow, E., et al. (2007). The β-propensity of Tau determines aggregation and synaptic loss in inducible mouse models of tauopathy. J Biol Chem 282, 31755–31765.
Elder, G.A., Gama Sosa, M.A., De Gasperi, R., Dickstein, D.L., and Hof, P. R. (2010). Presenilin transgenic mice as models of Alzheimer’s disease. Brain Struct Funct 214, 127–143.
Elhaik Goldman, S., Goez, D., Last, D., Naor, S., Liraz Zaltsman, S., Sharvit-Ginon, I., Atrakchi-Baranes, D., Shemesh, C., Twitto-Greenberg, R., Tsach, S., et al. (2018). High-fat diet protects the blood-brain barrier in an Alzheimer’s disease mouse model. Aging Cell 17, e12818.
Evans, C.E., Thomas, R.S., Freeman, T.J., Hvoslef-Eide, M., Good, M.A., and Kidd, E.J. (2019). Selective reduction of APP-BACE1 activity improves memory via NMDA-NR2B receptor-mediated mechanisms in aged PDAPP mice. Neurobiol Aging 75, 136–149.
Fan, R., Xu, F., Previti, M.L., Davis, J., Grande, A.M., Robinson, J.K., and Van Nostrand, W.E. (2007). Minocycline reduces microglial activation and improves behavioral deficits in a transgenic model of cerebral microvascular amyloid. J Neurosci 27, 3057–3063.
Fernandez-Funez, P., de Mena, L., and Rincon-Limas, D.E. (2015). Modeling the complex pathology of Alzheimer’s disease in Drosophila. Exp Neurol 274, 58–71.
Ferretti, M.T., Bruno, M.A., Ducatenzeiler, A., Klein, W.L., and Cuello, A. C. (2012). Intracellular Aβ-oligomers and early inflammation in a model of Alzheimer’s disease. Neurobiol Aging 33, 1329–1342.
Ferretti, M.T., Partridge, V.C., Leon, W., Canneva, F., Allard, S.N., Arvanitis, D., Vercauteren, F., Houle, D., Ducatenzeiler, A.L., Klein, W., et al. (2011). Transgenic mice as a model of pre-clinical Alzheimers disease. Curr Alzheimer Res 8, 4–23.
Flanigan, T.J., Xue, Y., Kishan Rao, S., Dhanushkodi, A., and McDonald, M.P. (2014). Abnormal vibrissa-related behavior and loss of barrel field inhibitory neurons in 5xFAD transgenics. Genes Brain Behav 13, 488–500.
Foster, J.B., Lashley, R., Zhao, F., Wang, X., Kung, N., Askwith, C.C., Lin, L., Shultis, M.W., Hodgetts, K.J., and Lin, C.L.G. (2019). Enhancement of tripartite synapses as a potential therapeutic strategy for Alzheimer’s disease: a preclinical study in rTg4510 mice. Alzheimers Res Ther 11, 75.
Frautschy, S.A., Yang, F., Irrizarry, M., Hyman, B., Saido, T.C., Hsiao, K., and Cole, G.M. (1998). Microglial response to amyloid plaques in APPsw transgenic mice. Am J Pathol 152, 307–317.
Friedrich, G., and Soriano, P. (1991). Promoter traps in embryonic stem cells: a genetic screen to identify and mutate developmental genes in mice. Genes Dev 5, 1513–1523.
Frost, J.L., Liu, B., Rahfeld, J.U., Kleinschmidt, M., O’Nuallain, B., Le, K. X., Lues, I., Caldarone, B.J., Schilling, S., Demuth, H.U., et al. (2015). An anti-pyroglutamate-3 Aβ vaccine reduces plaques and improves cognition in APPswe/PS1ΔE9 mice. Neurobiol Aging 36, 3187–3199.
Gaj, T., Gersbach, C.A., and Barbas Iii, C.F. (2013). ZFN, TALEN, and CRISPR/Cas-based methods for genome engineering. Trends Biotechnol 31, 397–405.
Gamage, K.K., and Kumar, S. (2017). Aducanumab therapy ameliorates calcium overload in a mouse model of Alzheimer’s disease. J Neurosci 37, 4430–4432.
Games, D., Adams, D., Alessandrini, R., Barbour, R., Borthelette, P., Blackwell, C., Carr, T., Clemens, J., Donaldson, T., Gillespie, F., et al. (1995). Alzheimer-type neuropathology in transgenic mice overexpressing V717F β-amyloid precursor protein. Nature 373, 523–527.
Gandy, S., Simon, A.J., Steele, J.W., Lublin, A.L., Lah, J.J., Walker, L.C., Levey, A.I., Krafft, G.A., Levy, E., Checler, F., et al. (2010). Days to criterion as an indicator of toxicity associated with human Alzheimer amyloid-β oligomers. Ann Neurol 68, 220–230.
Garcia-Alloza, M., Robbins, E.M., Zhang-Nunes, S.X., Purcell, S.M., Betensky, R.A., Raju, S., Prada, C., Greenberg, S.M., Bacskai, B.J., and Frosch, M.P. (2006). Characterization of amyloid deposition in the APPswe/PS1dE9 mouse model of Alzheimer disease. Neurobiol Dis 24, 516–524.
Garrick, D., Fiering, S., Martin, D.I.K., and Whitelaw, E. (1998). Repeat-induced gene silencing in mammals. Nat Genet 18, 56–59.
Gauthier, S., Wu, L., Rosa-Neto, P., and Jia, J. (2012). Prevention strategies for Alzheimer’s disease. Transl Neurodegener 1, 13.
Gelman, S., Palma, J., Tombaugh, G., and Ghavami, A. (2018). Differences in synaptic dysfunction between rTg4510 and APP/PS1 mouse models of Alzheimer’s disease. J Alzheimer Dis 61, 195–208.
Gengler, S., Hamilton, A., and Holscher, C. (2010). Synaptic plasticity in the hippocampus of a APP/PS1 mouse model of Alzheimer’s disease is impaired in old but not young mice. PLoS ONE 5, e9764.
Giannoni, P., Arango-Lievano, M., Neves, I.D., Rousset, M.C., Baranger, K., Rivera, S., Jeanneteau, F., Claeysen, S., and Marchi, N. (2016). Cerebrovascular pathology during the progression of experimental Alzheimer’s disease. Neurobiol Dis 88, 107–117.
Gjoneska, E., Pfenning, A.R., Mathys, H., Quon, G., Kundaje, A., Tsai, L. H., and Kellis, M. (2015). Conserved epigenomic signals in mice and humans reveal immune basis of Alzheimer’s disease. Nature 518, 365–369.
Goodwin, M.S., Sinyavskaya, O., Burg, F., O’Neal, V., Ceballos-Diaz, C., Cruz, P.E., Lewis, J., Giasson, B.I., Davies, P., Golde, T.E., et al. (2021). Anti-tau scFvs targeted to the cytoplasm or secretory pathway variably modify pathology and neurodegenerative phenotypes. Mol Ther 29, 859–872.
Gordon, M.N., Holcomb, L.A., Jantzen, P.T., DiCarlo, G., Wilcock, D., Boyett, K.W., Connor, K., Melachrino, J., O’Callaghan, J.P., and Morgan, D. (2002). Time course of the development of Alzheimer-like pathology in the doubly transgenic PS1+APP mouse. Exp Neurol 173, 183–195.
Götz, J., Bodea, L.G., and Goedert, M. (2018). Rodent models for Alzheimer disease. Nat Rev Neurosci 19, 583–598.
Govindarajan, N., Agis-Balboa, R.C., Walter, J., Sananbenesi, F., and Fischer, A. (2011). Sodium butyrate improves memory function in an Alzheimer’s disease mouse model when administered at an advanced stage of disease progression. J Alzheimer Dis 26, 187–197.
Gratuze, M., Leyns, C.E.G., Sauerbeck, A.D., St-Pierre, M.K., Xiong, M., Kim, N., Serrano, J.R., Tremblay, M.È., Kummer, T.T., Colonna, M., et al. (2020). Impact of TREM2R47H variant on tau pathology-induced gliosis and neurodegeneration. J Clin Invest 130, 4954–4968.
Grueninger, F., Bohrmann, B., Czech, C., Ballard, T.M., Frey, J.R., Weidensteiner, C., von Kienlin, M., and Ozmen, L. (2010). Phosphorylation of Tau at S422 is enhanced by Aβ in TauPS2APP triple transgenic mice. Neurobiol Dis 37, 294–306.
Gunawardena, S., and Goldstein, L.S.B. (2001). Disruption of axonal transport and neuronal viability by amyloid precursor protein mutations in Drosophila. Neuron 32, 389–401.
Guo, Q., Fu, W., Sopher, B.L., Miller, M.W., Ware, C.B., Martin, G.M., and Mattson, M.P. (1999). Increased vulnerability of hippocampal neurons to excitotoxic necrosis in presenilin-1 mutant knock-in mice. Nat Med 5, 101–106.
Hafezparast, M., Ahmad-Annuar, A., Wood, N.W., Tabrizi, S.J., and Fisher, E.M. (2002). Mouse models for neurological disease. Lancet Neurol 1, 215–224.
Hampton, D.W., Webber, D.J., Bilican, B., Goedert, M., Spillantini, M.G., and Chandran, S. (2010). Cell-mediated neuroprotection in a mouse model of human tauopathy. J Neurosci 30, 9973–9983.
Hara, M., Hirokawa, K., Kamei, S., and Uchihara, T. (2013). Isoform transition from four-repeat to three-repeat tau underlies dendrosomatic and regional progression of neurofibrillary pathology. Acta Neuropathol 125, 565–579.
Hartman, R.E., Izumi, Y., Bales, K.R., Paul, S.M., Wozniak, D.F., and Holtzman, D.M. (2005). Treatment with an amyloid-β antibody ameliorates plaque load, learning deficits, and hippocampal long-term potentiation in a mouse model of Alzheimer’s disease. J Neurosci 25, 6213–6220.
Hashimoto, S., Matsuba, Y., Kamano, N., Mihira, N., Sahara, N., Takano, J., Muramatsu, S., Saido, T.C., and Saito, T. (2019). Tau binding protein CAPON induces tau aggregation and neurodegeneration. Nat Commun 10, 2394.
Havas, D., Hutter-Paier, B., Ubhi, K., Rockenstein, E., Crailsheim, K., Masliah, E., and Windisch, M. (2011). A longitudinal study of behavioral deficits in an AβPP transgenic mouse model of Alzheimer’s disease. J Alzheimer Dis 25, 231–243.
He, A., Zhang, C., Ke, X., Yi, Y., Yu, Q., Zhang, T., Yu, H., Du, H., Li, H., Tian, Q., et al. (2022). VGLUT3 neurons in median raphe control the efficacy of spatial memory retrieval via ETV4 regulation of VGLUT3 transcription. Sci China Life Sci 65, 1590–1607.
Heckmann, B.L., Teubner, B.J.W., Boada-Romero, E., Tummers, B., Guy, C., Fitzgerald, P., Mayer, U., Carding, S., Zakharenko, S.S., Wileman, T., et al. (2020). Noncanonical function of an autophagy protein prevents spontaneous Alzheimer’s disease. Sci Adv 6, eabb9036.
Helboe, L., Egebjerg, J., Barkholt, P., and Volbracht, C. (2017). Early depletion of CA1 neurons and late neurodegeneration in a mouse tauopathy model. Brain Res 1665, 22–35.
Herzig, M.C., Winkler, D.T., Burgermeister, P., Pfeifer, M., Kohler, E., Schmidt, S.D., Danner, S., Abramowski, D., Stürchler-Pierrat, C., Bürki, K., et al. (2004). Aβ is targeted to the vasculature in a mouse model of hereditary cerebral hemorrhage with amyloidosis. Nat Neurosci 7, 954–960.
Heuer, E., F. Rosen, R., Cintron, A., and C. Walker, L. (2012). Nonhuman primate models of Alzheimer-like cerebral proteopathy. Curr Pharm Des 18, 1159–1169.
Holcomb, L., Gordon, M.N., McGowan, E., Yu, X., Benkovic, S., Jantzen, P., Wright, K., Saad, I., Mueller, R., Morgan, D., et al. (1998). Accelerated Alzheimer-type phenotype in transgenic mice carrying both mutant amyloid precursor protein and presenilin 1 transgenes. Nat Med 4, 97–100.
Hole, K.L., Staniaszek, L.E., Menon Balan, G., Mason, J.M., Brown, J.T., and Williams, R.J. (2021). Oral (−)-epicatechin inhibits progressive tau pathology in rTg4510 mice independent of direct actions at GSK3β. Front Neurosci 15, 697319.
Hong, S., Beja-Glasser, V.F., Nfonoyim, B.M., Frouin, A., Li, S., Ramakrishnan, S., Merry, K.M., Shi, Q., Rosenthal, A., Barres, B.A., et al. (2016). Complement and microglia mediate early synapse loss in Alzheimer mouse models. Science 352, 712–716.
Hoover, B.R., Reed, M.N., Su, J., Penrod, R.D., Kotilinek, L.A., Grant, M. K., Pitstick, R., Carlson, G.A., Lanier, L.M., Yuan, L.L., et al. (2010). Tau mislocalization to dendritic spines mediates synaptic dysfunction independently of neurodegeneration. Neuron 68, 1067–1081.
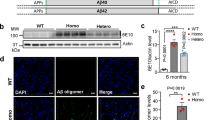
Hou, T. T., Yang, H. Y., Wang, W., Wu, Q. Q., Tian, Y. R., and Jia, J. P. (2018). Sulforaphane inhibits the generation of amyloid-β oligomer and promotes spatial learning and memory in Alzheimer’s disease (PS1V97L) transgenic mice. J Alzheimer Dis 62, 1803–1813.
Howlett, D.R., Bowler, K., Soden, P.E., Riddell, D., Davis, J.B., Richardson, J.C., Burbidge, S.A., Gonzalez, M.I., Irving, E.A., Lawman, A., et al. (2008). Abeta deposition and related pathology in an APP x PS1 transgenic mouse model of Alzheimer’s disease. Histol Histopathol 23, 67–76.
Howlett, D.R., Richardson, J.C., Austin, A., Parsons, A.A., Bate, S.T., Davies, D.C., and Gonzalez, M.I. (2004). Cognitive correlates of Aβ deposition in male and female mice bearing amyloid precursor protein and presenilin-1 mutant transgenes. Brain Res 1017, 130–136.
Hsia, A.Y., Masliah, E., McConlogue, L., Yu, G.Q., Tatsuno, G., Hu, K., Kholodenko, D., Malenka, R.C., Nicoll, R.A., and Mucke, L. (1999). Plaque-independent disruption of neural circuits in Alzheimer’s disease mouse models. Proc Natl Acad Sci USA 96, 3228–3233.
Hsiao, K., Chapman, P., Nilsen, S., Eckman, C., Harigaya, Y., Younkin, S., Yang, F., and Cole, G. (1996). Correlative memory deficits, Aβ elevation, and amyloid plaques in transgenic mice. Science 274, 99–103.
Hu, X., Das, B., Hou, H., He, W., and Yan, R. (2018). BACE1 deletion in the adult mouse reverses preformed amyloid deposition and improves cognitive functions. J Exp Med 215, 927–940.
Huang, K., Marcora, E., Pimenova, A.A., Di Narzo, A.F., Kapoor, M., Jin, S.C., Harari, O., Bertelsen, S., Fairfax, B.P., Czajkowski, J., et al. (2017). A common haplotype lowers PU.1 expression in myeloid cells and delays onset of Alzheimer’s disease. Nat Neurosci 20, 1052–1061.
Hwang, D.Y., Chae, K.R., Kang, T.S., Hwang, J.H., Lim, C.H., Kang, H.K., Goo, J.S., Lee, M.R., Lim, H.J., Min, S.H., et al. (2002). Alterations in behavior, amyloid p-42, caspase-3, and Cox-2 in mutant PS2 transgenic mouse model of Alzheimer’s disease. FASEB J 16, 805–813.
Hwang, D.Y., Cho, J.S., Lee, S.H., Chae, K.R., Lim, H.J., Min, S.H., Seo, S.J., Song, Y.S., Song, C.W., Paik, S.G., et al. (2004). Aberrant expressions of pathogenic phenotype in Alzheimer’s diseased transgenic mice carrying NSE-controlled APPsw. Exp Neurol 186, 20–32.
Hyman, B., and Tanzi, R.E. (2019). Effects of species-specific genetics on Alzheimer’s mouse models. Neuron 101, 351–352.
Irizarry, M.C., Mcnamara, M., Fedorchak, K., Hsiao, K., and Hyman, B.T. (1997). APPSW transgenic mice develop age-related Aβ deposits and neuropil abnormalities, but no neuronal loss in CA1. J Neuropathol Exp Neurol 56, 965–973.
Jackson, H.M., Soto, I., Graham, L.C., Carter, G.W., and Howell, G.R. (2013). Clustering of transcriptional profiles identifies changes to insulin signaling as an early event in a mouse model of Alzheimer’s disease. BMC Genomics 14, 831.
Jackson, R.J., Rudinskiy, N., Herrmann, A.G., Croft, S., Kim, J.M., Petrova, V., Ramos-Rodriguez, J.J., Pitstick, R., Wegmann, S., Garcia-Alloza, M., Carlson, G.A., Hyman, B.T., and Spires-Jones, T.L. (2016). Human tau increases amyloid beta plaque size but not amyloid betamediated synapse loss in a novel mouse model of Alzheimer’s disease. Eur J Neurosci 44, 3056–3066.
Jacobsen, J.S., Wu, C.C., Redwine, J.M., Comery, T.A., Arias, R., Bowlby, M., Martone, R., Morrison, J.H., Pangalos, M.N., Reinhart, P.H., et al. (2006). Early-onset behavioral and synaptic deficits in a mouse model of Alzheimer’s disease. Proc Natl Acad Sci USA 103, 5161–5166.
Jaenisch, R., and Mintz, B. (1974). Simian virus 40 DNA sequences in DNA of healthy adult mice derived from preimplantation blastocysts injected with viral DNA. Proc Natl Acad Sci USA 71, 1250–1254.
Jankowsky, J.L., Fadale, D.J., Anderson, J., Xu, G.M., Gonzales, V., Jenkins, N.A., Copeland, N.G., Lee, M.K., Younkin, L.H., Wagner, S. L., et al. (2004). Mutant presenilins specifically elevate the levels of the 42 residue β-amyloid peptide in vivo: evidence for augmentation of a 42-specific γ secretase. Hum Mol Genet 13, 159–170.
Jankowsky, J.L., Slunt, H.H., Gonzales, V., Savonenko, A.V., Wen, J.C., Jenkins, N.A., Copeland, N.G., Younkin, L.H., Lester, H.A., Younkin, S.G., et al. (2005). Persistent amyloidosis following suppression of Abeta production in a transgenic model of Alzheimer disease. PLoS Med 2, e355.
Jankowsky, J.L., Slunt, H.H., Ratovitski, T., Jenkins, N.A., Copeland, N.G., and Borchelt, D.R. (2001). Co-expression of multiple transgenes in mouse CNS: a comparison of strategies. Biomol Eng 17, 157–165.
Jawhar, S., Trawicka, A., Jenneckens, C., Bayer, T.A., and Wirths, O. (2012). Motor deficits, neuron loss, and reduced anxiety coinciding with axonal degeneration and intraneuronal Aβ aggregation in the 5XFAD mouse model of Alzheimer’s disease. Neurobiol Aging 33, 196.e29–196.e40.
Jay, T.R., Hirsch, A.M., Broihier, M.L., Miller, C.M., Neilson, L.E., Ransohoff, R.M., Lamb, B.T., and Landreth, G.E. (2017). Disease progression-dependent effects of TREM2 deficiency in a mouse model of Alzheimer’s disease. J Neurosci 37, 637–647.
Jia, J., Xu, E., Shao, Y., Jia, J., Sun, Y., and Li, D. (2005). One novel presenilin-1 gene mutation in a Chinese pedigree of familial Alzheimer’s disease. J Alzheimer Dis 7, 119–124; discussion 173–180.
Jia, L., Du, Y., Chu, L., Zhang, Z., Li, F., Lyu, D., Li, Y., Li, Y., Zhu, M., Jiao, H., et al. (2020a). Prevalence, risk factors, and management of dementia and mild cognitive impairment in adults aged 60 years or older in China: a cross-sectional study. Lancet Public Health 5, e661–e671.
Jia, L., Fu, Y., Shen, L., Zhang, H., Zhu, M., Qiu, Q., Wang, Q., Yan, X., Kong, C., Hao, J., et al. (2020b). PSEN1, PSEN2, and APP mutations in 404 Chinese pedigrees with familial Alzheimer’s disease. Alzheimers Dement 16, 178–191.
Jia, L., Quan, M., Fu, Y., Zhao, T., Li, Y., Wei, C., Tang, Y., Qin, Q., Wang, F., Qiao, Y., et al. (2020c). Dementia in China: epidemiology, clinical management, and research advances. Lancet Neurol 19, 81–92.
Jiao, H., and Jia, J. (2022). Ginsenoside compound K acts via LRP1 to alleviate Amyloid β42-induced neuroinflammation in microglia by suppressing NF-κB. Biochem Biophys Res Commun 590, 14–19.
Josephine Boder, E., and Banerjee, I.A. (2021). Alzheimer’s disease: current perspectives and advances in physiological modeling. Bioengineering 8, 211.
Kamphuis, W., Mamber, C., Moeton, M., Kooijman, L., Sluijs, J.A., Jansen, A.H., Verveer, M., de Groot, L.R., Smith, V.D., Rangarajan, S., et al. (2012). GFAP isoforms in adult mouse brain with a focus on neurogenic astrocytes and reactive astrogliosis in mouse models of Alzheimer disease. PLoS ONE 7, e42823.
Kang, S., Kim, J., and Chang, K.A. (2021). Spatial memory deficiency early in 6xTg Alzheimer’s disease mouse model. Sci Rep 11, 1334.
Karran, E., and De Strooper, B. (2022). The amyloid hypothesis in Alzheimer disease: new insights from new therapeutics. Nat Rev Drug Discov 21, 306–318.
Karran, E., and Hardy, J. (2014). A critique of the drug discovery and phase 3 clinical programs targeting the amyloid hypothesis for Alzheimer disease. Ann Neurol 76, 185–205.
Kawasumi, M., Chiba, T., Yamada, M., Miyamae-Kaneko, M., Matsuoka, M., Nakahara, J., Tomita, T., Iwatsubo, T., Kato, S., Aiso, S., et al. (2004). Targeted introduction of V642I mutation in amyloid precursor protein gene causes functional abnormality resembling early stage of Alzheimer’s disease in aged mice. Eur J Neurosci 19, 2826–2838.
Kelly, P.H., Bondolfi, L., Hunziker, D., Schlecht, H.P., Carver, K., Maguire, E., Abramowski, D., Wiederhold, K.H., Sturchler-Pierrat, C., Jucker, M., et al. (2003). Progressive age-related impairment of cognitive behavior in APP23 transgenic mice. Neurobiol Aging 24, 365–378.
Keren-Shaul, H., Spinrad, A., Weiner, A., Matcovitch-Natan, O., Dvir-Szternfeld, R., Ulland, T.K., David, E., Baruch, K., Lara-Astaiso, D., Toth, B., et al. (2017). A unique microglia type associated with restricting development of Alzheimer’s disease. Cell 169, 1276–1290. e17.
Kim, J., and Jeong, Y. (2015). In vivo image of cerebral amyloid angiopathy in an Alzheimer’s disease mouse model. J Stroke 17, 87–88.
Kim, J.H., Nam, Y.P., Jeon, S.M., Han, H.S., and Suk, K. (2012). Amyloid neurotoxicity is attenuated by metallothionein: dual mechanisms at work. J Neurochem 121, 751–762.
Kimura, R., MacTavish, D., Yang, J., Westaway, D., and Jhamandas, J.H. (2012). Beta amyloid-induced depression of hippocampal long-term potentiation is mediated through the amylin receptor. J Neurosci 32, 17401–17406.
Kimura, R., and Ohno, M. (2009). Impairments in remote memory stabilization precede hippocampal synaptic and cognitive failures in 5XFAD Alzheimer mouse model. Neurobiol Dis 33, 229–235.
King, A. (2018). The search for better animal models of Alzheimer’s disease. Nature 559, S13–S15.
Klyubin, I., Walsh, D.M., Lemere, C.A., Cullen, W.K., Shankar, G.M., Betts, V., Spooner, E.T., Jiang, L., Anwyl, R., Selkoe, D.J., et al. (2005). Amyloid β protein immunotherapy neutralizes Aβ oligomers that disrupt synaptic plasticity in vivo. Nat Med 11, 556–561.
Knobloch, M., Farinelli, M., Konietzko, U., Nitsch, R.M., and Mansuy, I. M. (2007a). Aβ oligomer-mediated long-term potentiation impairment involves protein phosphatase 1-dependent mechanisms. J Neurosci 27, 7648–7653.
Knobloch, M., Konietzko, U., Krebs, D.C., and Nitsch, R.M. (2007b). Intracellular Aβ and cognitive deficits precede β-amyloid deposition in transgenic arcAβ mice. Neurobiol Aging 28, 1297–1306.
Kopeikina, K.J., Polydoro, M., Tai, H.C., Yaeger, E., Carlson, G.A., Pitstick, R., Hyman, B.T., and Spires-Jones, T.L. (2013). Synaptic alterations in the rTg4510 mouse model of tauopathy. J Comp Neurol 521, 1334–1353.
Kulnane, L.S., and Lamb, B.T. (2001). Neuropathological characterization of mutant amyloid precursor protein yeast artificial chromosome transgenic mice. Neurobiol Dis 8, 982–992.
Kurt, M.A., Davies, D.C., Kidd, M., Duff, K., and Howlett, D.R. (2003). Hyperphosphorylated tau and paired helical filament-like structures in the brains of mice carrying mutant amyloid precursor protein and mutant presenilin-1 transgenes. Neurobiol Dis 14, 89–97.
Kwan, P., Ho, A., and Baum, L. (2022). Effects of deferasirox in Alzheimer’s disease and tauopathy animal models. Biomolecules 12, 365.
Lalonde, R., Kim, H.D., Maxwell, J.A., and Fukuchi, K. (2005). Exploratory activity and spatial learning in 12-month-old APP695SWE/co+PS1/ΔE9 mice with amyloid plaques. Neurosci Lett 390, 87–92.
Lalonde, R., and Strazielle, C. (2005). PS1 knockin mice with the Japanese I213T mutation: effects on exploratory activity, motor coordination, and spatial learning. Behav Brain Res 162, 182–190.
Lamb, B.A., Bardel, K.A., Kulnane, L.S., Anderson, J.J., Holtz, G., Wagner, S.L., Sisodia, S.S., and Hoeger, E.J. (1999). Amyloid production and deposition in mutant amyloid precursor protein and presenilin-1 yeast artificial chromosome transgenic mice. Nat Neurosci 2, 695–697.
Lamb, B.T., Call, L.M., Slunt, H.H., Bardel, K.A., Lawler, A.M., Eckman, C.B., Younkin, S.G., Holtz, G., Wagner, S.L., Price, D.L., et al. (1997). Altered metabolism of familial Alzheimer’s disease-linked amyloid precursor protein variants in yeast artificial chromosome transgenic mice. Hum Mol Genet 6, 1535–1541.
Lambert, M.P., Velasco, P.T., Chang, L., Viola, K.L., Fernandez, S., Lacor, P.N., Khuon, D., Gong, Y., Bigio, E.H., Shaw, P., et al. (2007). Monoclonal antibodies that target pathological assemblies of Aβ. J Neurochem 100, 23–35.
Lanz, T.A., Carter, D.B., and Merchant, K.M. (2003). Dendritic spine loss in the hippocampus of young PDAPP and Tg2576 mice and its prevention by the ApoE2 genotype. Neurobiol Dis 13, 246–253.
Larson, J., Lynch, G., Games, D., and Seubert, P. (1999). Alterations in synaptic transmission and long-term potentiation in hippocampal slices from young and aged PDAPP mice. Brain Res 840, 23–35.
Lasagna-Reeves, C.A., de Haro, M., Hao, S., Park, J., Rousseaux, M.W.C., Al-Ramahi, I., Jafar-Nejad, P., Vilanova-Velez, L., See, L., De Maio, A., et al. (2016). Reduction of Nuak1 decreases tau and reverses phenotypes in a tauopathy mouse model. Neuron 92, 407–418.
Lee, C.Y.D., Daggett, A., Gu, X., Jiang, L.L., Langfelder, P., Li, X., Wang, N., Zhao, Y., Park, C.S., Cooper, Y., et al. (2018). Elevated TREM2 gene dosage reprograms microglia responsivity and ameliorates pathological phenotypes in Alzheimer’s disease models. Neuron 97, 1032–1048.e5.
Lee, H.J., Jeon, S.G., Kim, J., Kang, R.J., Kim, S.M., Han, K.M., Park, H., Kim, K.T., Sung, Y.M., Nam, H.Y., et al. (2021a). Ibrutinib modulates Aβ/tau pathology, neuroinflammation, and cognitive function in mouse models of Alzheimer’s disease. Aging Cell 20, e13332.
Lee, H.S., Kim, M.W., Jin, K.S., Shin, H.C., Kim, W.K., Lee, S.C., Kim, S. J., Lee, E.W., and Ku, B. (2021b). Molecular analysis of the interaction between human PTPN21 and the oncoprotein E7 from human papillomavirus genotype 18. MolCells 44, 26–37.
Lefterov, I., Fitz, N.F., Cronican, A., Lefterov, P., Staufenbiel, M., and Koldamova, R. (2009). Memory deficits in APP23/Abca1+/− mice correlate with the level of Aβ oligomers. ASN Neuro 1, AN20090015.
Lehman, E.J.H., Kulnane, L.S., and Lamb, B.T. (2003). Alterations in β-amyloid production and deposition in brain regions of two transgenic models. Neurobiol Aging 24, 645–653.
Leinenga, G., Koh, W.K., and Götz, J. (2021). A comparative study of the effects of Aducanumab and scanning ultrasound on amyloid plaques and behavior in the APP23 mouse model of Alzheimer disease. Alzheimers Res Ther 13, 76.
Leon, W.C., Canneva, F., Partridge, V., Allard, S., Ferretti, M.T., DeWilde, A., Vercauteren, F., Atifeh, R., Ducatenzeiler, A., Klein, W., et al. (2010). A novel transgenic rat model with a full Alzheimer’s-like amyloid pathology displays pre-plaque intracellular amyloid-β-associated cognitive impairment. J Alzheimer Dis 20, 113–126.
Lewis, J., Dickson, D.W., Lin, W.L., Chisholm, L., Corral, A., Jones, G., Yen, S.H., Sahara, N., Skipper, L., Yager, D., et al. (2001). Enhanced neurofibrillary degeneration in transgenic mice expressing mutant tau and APP. Science 293, 1487–1491.
Lewis, J., McGowan, E., Rockwood, J., Melrose, H., Nacharaju, P., Van Slegtenhorst, M., Gwinn-Hardy, K., Murphy, M.P., Baker, M., Yu, X., et al. (2000). Neurofibrillary tangles, amyotrophy and progressive motor disturbance in mice expressing mutant (P301L) tau protein. Nat Genet 25, 402–405.
Li, B., Xu, L., Li, F., Li, Y., Zhao, Y., Zhang, H., Quan, M., and Jia, J. (2022). CaMKIIα signaling is required for the neuroprotective effects of Dl-3-n-butylphthalide in Alzheimer’s disease. Mol Neurobiol 59, 3370–3381.
Li, D., Qiu, Z., Shao, Y., Chen, Y., Guan, Y., Liu, M., Li, Y., Gao, N., Wang, L., Lu, X., et al. (2013). Heritable gene targeting in the mouse and rat using a CRISPR-Cas system. Nat Biotechnol 31, 681–683.
Li, F., Wu, X., Li, J., and Niu, Q. (2016). Ginsenoside Rg1 ameliorates hippocampal long-term potentiation and memory in an Alzheimer’s disease model. Mol Med Rep 13, 4904–4910.
Li, H., Jia, J., Wang, W., Hou, T., Tian, Y., Wu, Q., Xu, L., Wei, Y., and Wang, X. (2018). Honokiol alleviates cognitive deficits of Alzheimer’s disease (PS1V97L) transgenic mice by activating mitochondrial SIRT3. J Alzheimer Dis 64, 291–302.
Li, H.Q., Ip, S.P., Yuan, Q.J., Zheng, G.Q., Tsim, K.K.W., Dong, T.T.X., Lin, G., Han, Y., Liu, Y., Xian, Y.F., et al. (2019). Isorhynchophylline ameliorates cognitive impairment via modulating amyloid pathology, tau hyperphosphorylation and neuroinflammation: Studies in a transgenic mouse model of Alzheimer’s disease. Brain Behav Immun 82, 264–278.
Li, S., Wu, Z., and Le, W. (2021a). Traditional Chinese medicine for dementia. Alzheimers Dement 17, 1066–1071.
Li, T., Martin, E., Abada, Y., Boucher, C., Cès, A., Youssef, I., Fenaux, G., Forand, Y., Legrand, A., Nachiket, N., et al. (2020). Effects of chronic masitinib treatment in APPswe/PSEN1dE9 transgenic mice modeling Alzheimer’s disease. J Alzheimer Dis 76, 1339–1345.
Li, W., Pang, Y., Wang, Y., Mei, F., Guo, M., Wei, Y., Li, X., Qin, W., Wang, W., Jia, L., et al. (2023). Aberrant palmitoylation caused by a ZDHHC21 mutation contributes to pathophysiology of Alzheimer’s disease. BMC Med 21, 223.
Li, W., Wang, S., Zhang, H., Li, B., Xu, L., Li, Y., Kong, C., Jiao, H., Wang, Y., Pang, Y., et al. (2021b). Honokiol restores microglial phagocytosis by reversing metabolic reprogramming. J Alzheimers Dis 82, 1475–1485.
Lillehaug, S., Syverstad, G.H., Nilsson, L.N.G., Bjaalie, J.G., Leergaard, T. B., and Torp, R. (2014). Brainwide distribution and variance of amyloid-beta deposits in tg-ArcSwe mice. Neurobiol Aging 35, 556–564.
Liu, J., Baum, L., Yu, S., Lin, Y., Xiong, G., Chang, R.C.C., So, K.F., and Chiu, K. (2021a). Preservation of retinal function through synaptic stabilization in Alzheimer’s disease model mouse retina by lycium barbarum extracts. Front Aging Neurosci 13, 788798.
Liu, P., Paulson, J.B., Forster, C.L., Shapiro, S.L., Ashe, K.H., and Zahs, K. R. (2015). Characterization of a novel mouse model of Alzheimer’s disease—amyloid pathology and unique β-amyloid oligomer profile. PLoS ONE 10, e0126317.
Liu, W., Li, Y., Li, Y., Xu, L., and Jia, J. (2023). Carnosic acid attenuates AβOs-induced apoptosis and synaptic impairment via regulating NMDAR2B and its downstream cascades in SH-SY5Y cells. Mol Neurobiol 60, 133–144.
Liu, X., Zhou, Q., Zhang, J.H., Wang, K.Y., Saito, T., Saido, T.C., Wang, X., Gao, X., and Azuma, K. (2021b). Microglia-based sex-biased neuropathology in early-stage Alzheimer’s disease model mice and the potential pharmacologic efficacy of dioscin. Cells 10, 3261.
Liu, Y., Yao, J., Song, Z., Guo, W., Sun, B., Wei, J., Estillore, J.P., Back, T. G., and Chen, S.R.W. (2021c). Limiting RyR2 open time prevents Alzheimer’s disease-related deficits in the 3xTG-AD mouse model. J Neurosci Res 99, 2906–2921.
Lo, A.C., Tesseur, I., Scopes, D.I.C., Nerou, E., Callaerts-Vegh, Z., Vermaercke, B., Treherne, J.M., De Strooper, B., and D’Hooge, R. (2013). Dose-dependent improvements in learning and memory deficits in APPPS1-21 transgenic mice treated with the orally active Aβ toxicity inhibitor SEN1500. Neuropharmacology 75, 458–466.
Long, J.M., and Holtzman, D.M. (2019). Alzheimer disease: an update on pathobiology and treatment strategies. Cell 179, 312–339.
Lord, A., Englund, H., Söderberg, L., Tucker, S., Clausen, F., Hillered, L., Gordon, M., Morgan, D., Lannfelt, L., Pettersson, F.E., et al. (2009). Amyloid-β protofibril levels correlate with spatial learning in Arctic Alzheimer’s disease transgenic mice. FEBS J 276, 995–1006.
Lord, A., Kalimo, H., Eckman, C., Zhang, X.Q., Lannfelt, L., and Nilsson, L.N.G. (2006). The Arctic Alzheimer mutation facilitates early intraneuronal Aβ aggregation and senile plaque formation in transgenic mice. Neurobiol Aging 27, 67–77.
Lord, A., Philipson, O., Klingstedt, T., Westermark, G., Hammarström, P., Nilsson, K.P.R., and Nilsson, L.N.G. (2011). Observations in APP bitransgenic mice suggest that diffuse and compact plaques form via independent processes in Alzheimer’s disease. Am J Pathol 178, 2286–2298.
Lyu, D., and Jia, J. (2022). Cryptotanshinone attenuates amyloid-β42-induced tau phosphorylation by regulating PI3K/Akt/GSK3β pathway in HT22 cells. Mol Neurobiol 59, 4488–4500.
Maeda, S., Djukic, B., Taneja, P., Yu, G., Lo, I., Davis, A., Craft, R., Guo, W., Wang, X., Kim, D., et al. (2016). Expression of A152T human tau causes age-dependent neuronal dysfunction and loss in transgenic mice. EMBO Rep 17, 530–551.
Malm, T.M., Iivonen, H., Goldsteins, G., Keksa-Goldsteine, V., Ahtoniemi, T., Kanninen, K., Salminen, A., Auriola, S., Van Groen, T., Tanila, H., et al. (2007). Pyrrolidine dithiocarbamate activates Akt and improves spatial learning in APP/PS1 mice without affecting β-amyloid burden. J Neurosci 27, 3712–3721.
Marazuela, P., Paez-Montserrat, B., Bonaterra-Pastra, A., Solé, M., and Hernández-Guillamon, M. (2022). Impact of cerebral amyloid angiopathy in two transgenic mouse models of cerebral β-amyloidosis: a neuropathological study. Int J Mol Sci 23, 4972.
Martini, A.C., Forner, S., Trujillo-Estrada, L., Baglietto-Vargas, D., and LaFerla, F.M. (2018). Past to future: what animal models have taught us about Alzheimer’s disease. J Alzheimers Dis 64, S365–S378.
Masliah, E., Sisk, A., Mallory, M., and Games, D. (2001). Neurofibrillary pathology in transgenic mice overexpressing V717F β-amyloid precursor protein. J Neuropathol Exp Neurol 60, 357–368.
Maurin, H., Chong, S.A., Kraev, I., Davies, H., Kremer, A., Seymour, C. M., Lechat, B., Jaworski, T., Borghgraef, P., Devijver, H., et al. (2014). Early structural and functional defects in synapses and myelinated axons in stratum lacunosum moleculare in two preclinical models for tauopathy. PLoS ONE 9, e87605.
McGowan, E., Sanders, S., Iwatsubo, T., Takeuchi, A., Saido, T., Zehr, C., Yu, X., Uljon, S., Wang, R., Mann, D., et al. (1999). Amyloid phenotype characterization of transgenic mice overexpressing both mutant amyloid precursor protein and mutant presenilin 1 transgenes. Neurobiol Dis 6, 231–244.
Mckean, N.E., Handley, R.R., and Snell, R.G. (2021). A review of the current mammalian models of Alzheimer’s disease and challenges that need to be overcome. Int J Mol Sci 22, 13168.
Mehla, J., Lacoursiere, S.G., Lapointe, V., McNaughton, B.L., Sutherland, R.J., McDonald, R.J., and Mohajerani, M.H. (2019). Age-dependent behavioral and biochemical characterization of single APP knock-in mouse (APPNL-G-F/NL-G-F) model of Alzheimer’s disease. Neurobiol Aging 75, 25–37.
Mei, Z., Zhang, F., Tao, L., Zheng, W., Cao, Y., Wang, Z., Tang, S., Le, K., Chen, S., Pi, R., et al. (2009). Cryptotanshinone, a compound from Salvia miltiorrhiza modulates amyloid precursor protein metabolism and attenuates β-amyloid deposition through upregulating α-secretase in vivo and in vitro. Neurosci Lett 452, 90–95.
Meilandt, W.J., Maloney, J.A., Imperio, J., Lalehzadeh, G., Earr, T., Crowell, S., Bainbridge, T.W., Lu, Y., Ernst, J.A., Fuji, R.N., et al. (2019). Characterization of the selective in vitro and in vivo binding properties of crenezumab to oligomeric Aβ. Alzheimers Res Ther 11, 97.
Meilandt, W.J., Ngu, H., Gogineni, A., Lalehzadeh, G., Lee, S.H., Srinivasan, K., Imperio, J., Wu, T., Weber, M., Kruse, A.J., et al. (2020). Trem2 deletion reduces late-stage amyloid plaque accumulation, elevates the Aβ42:Aβ40 ratio, and exacerbates axonal dystrophy and dendritic spine loss in the PS2APP Alzheimer’s mouse model. J Neurosci 40, 1956–1974.
Miao, J., Vitek, M.P., Xu, F., Previti, M.L., Davis, J., and Van Nostrand, W. E. (2005a). Reducing cerebral microvascular amyloid-β protein deposition diminishes regional neuroinflammation in vasculotropic mutant amyloid precursor protein transgenic mice. J Neurosci 25, 6271–6277.
Miao, J., Xu, F., Davis, J., Otte-Höller, I., Verbeek, M.M., and Van Nostrand, W.E. (2005b). Cerebral microvascular amyloid β protein deposition induces vascular degeneration and neuroinflammation in transgenic mice expressing human vasculotropic mutant amyloid β precursor protein. Am J Pathol 167, 505–515.
Migliore, L., and Coppedè, F. (2022). Gene-environment interactions in Alzheimer disease: the emerging role of epigenetics. Nat Rev Neurol 18, 643–660.
Minkeviciene, R., Ihalainen, J., Malm, T., Matilainen, O., Keksa-Goldsteine, V., Goldsteins, G., Iivonen, H., Leguit, N., Glennon, J., Koistinaho, J., et al. (2008). Age-related decrease in stimulated glutamate release and vesicular glutamate transporters in APP/PS1 transgenic and wild-type mice. J Neurochem 105, 584–594.
Mocanu, M.M., Nissen, A., Eckermann, K., Khlistunova, I., Biernat, J., Drexler, D., Petrova, O., Schönig, K., Bujard, H., Mandelkow, E., et al. (2008). The potential for β-structure in the repeat domain of tau protein determines aggregation, synaptic decay, neuronal loss, and coassembly with endogenous tau in inducible mouse models of tauopathy. J Neurosci 28, 737–748.
Moechars, D., Dewachter, I., Lorent, K., Reversé, D., Baekelandt, V., Naidu, A., Tesseur, I., Spittaels, K., Haute, C.V.D., Checler, F., et al. (1999). Early phenotypic changes in transgenic mice that overexpress different mutants of amyloid precursor protein in brain. J Biol Chem 274, 6483–6492.
Mucke, L., Masliah, E., Yu, G.Q., Mallory, M., Rockenstein, E.M., Tatsuno, G., Hu, K., Kholodenko, D., Johnson-Wood, K., and McConlogue, L. (2000). High-level neuronal expression of Aβ1–42 in wild-type human amyloid protein precursor transgenic mice: synaptotoxicity without plaque formation. J Neurosci 20, 4050–4058.
Mullan, M., Crawford, F., Axelman, K., Houlden, H., Lilius, L., Winblad, B., and Lannfelt, L. (1992). A pathogenic mutation for probable Alzheimer’s disease in the APP gene at the N-terminus of β-amyloid. Nat Genet 1, 345–347.
Mullane, K., and Williams, M. (2019). Preclinical models of Alzheimer’s disease: relevance and translational validity. Curr Protoc Pharmacol 84, e57.
Müller-Schiffmann, A., Herring, A., Abdel-Hafiz, L., Chepkova, A.N., Schäble, S., Wedel, D., Horn, A.H.C., Sticht, H., de Souza Silva, M.A., Gottmann, K., et al. (2016). Amyloid-β dimers in the absence of plaque pathology impair learning and synaptic plasticity. Brain 139, 509–525.
Murata, N., Murakami, K., Ozawa, Y., Kinoshita, N., Irie, K., Shirasawa, T., and Shimizu, T. (2010). Silymarin attenuated the amyloid β plaque burden and improved behavioral abnormalities in an Alzheimer’s disease mouse model. Biosci Biotechnol Biochem 74, 2299–2306.
Nagakura, A., Shitaka, Y., Yarimizu, J., and Matsuoka, N. (2013). Characterization of cognitive deficits in a transgenic mouse model of Alzheimer’s disease and effects of donepezil and memantine. Eur J Pharmacol 703, 53–61.
Nakano, Y., Kondoh, G., Kudo, T., Imaizumi, K., Kato, M., Miyazaki, J., Tohyama, M., Takeda, J., and Takeda, M. (1999). Accumulation of murine amyloidbeta42 in a gene-dosage-dependent manner in PS1 ‘knock-in’ mice. Eur J Neurosci 11, 2577–2581.
Nedelec, T., Couvy-Duchesne, B., Monnet, F., Daly, T., Ansart, M., Gantzer, L., Lekens, B., Epelbaum, S., Dufouil, C., and Durrleman, S. (2022). Identifying health conditions associated with Alzheimer’s disease up to 15 years before diagnosis: an agnostic study of French and British health records. Lancet Digital Health 4, e169–e178.
Nilsson, P., Saito, T., and Saido, T.C. (2014). New mouse model of Alzheimer’s. ACS Chem Neurosci 5, 499–502.
Nott, A., Holtman, I.R., Coufal, N.G., Schlachetzki, J.C.M., Yu, M., Hu, R., Han, C.Z., Pena, M., Xiao, J., Wu, Y., et al. (2019). Brain cell type-specific enhancer-promoter interactome maps and disease-risk association. Science 366, 1134–1139.
O’Leary, T.P., Robertson, A., Chipman, P.H., Rafuse, V.F., and Brown, R.E. (2018). Motor function deficits in the 12 month-old female 5xFAD mouse model of Alzheimer’s disease. Behav Brain Res 337, 256–263.
O’Leary, T.P., Shin, S., Fertan, E., Dingle, R.N., Almuklass, A., Gunn, R. K., Yu, Z., Wang, J., and Brown, R.E. (2017). Reduced acoustic startle response and peripheral hearing loss in the 5xFAD mouse model of Alzheimer’s disease. Genes Brain Behav 16, 554–563.
Oakley, H., Cole, S.L., Logan, S., Maus, E., Shao, P., Craft, J., Guillozet-Bongaarts, A., Ohno, M., Disterhoft, J., Van Eldik, L., et al. (2006). Intraneuronal β-amyloid aggregates, neurodegeneration, and neuron loss in transgenic mice with five familial Alzheimer’s disease mutations: potential factors in amyloid plaque formation. J Neurosci 26, 10129–10140.
Oddo, S., Caccamo, A., Shepherd, J.D., Murphy, M.P., Golde, T.E., Kayed, R., Metherate, R., Mattson, M.P., Akbari, Y., and LaFerla, F.M. (2003). Triple-transgenic model of Alzheimer’s disease with plaques and tangles. Neuron 39, 409–421.
Oddo, S., Caccamo, A., Tran, L., Lambert, M.P., Glabe, C.G., Klein, W.L., and LaFerla, F.M. (2006). Temporal profile of amyloid-β (Aβ) oligomerization in an in vivo model of Alzheimer disease. J Biol Chem 281, 1599–1604.
Ohno, M., Chang, L., Tseng, W., Oakley, H., Citron, M., Klein, W.L., Vassar, R., and Disterhoft, J.F. (2006). Temporal memory deficits in Alzheimer’s mouse models: rescue by genetic deletion of BACE1. Eur J Neurosci 23, 251–260.
Onos, K.D., Uyar, A., Keezer, K.J., Jackson, H.M., Preuss, C., Acklin, C.J., O’Rourke, R., Buchanan, R., Cossette, T.L., Sukoff Rizzo, S.J., et al. (2019). Enhancing face validity of mouse models of Alzheimer’s disease with natural genetic variation. PLoS Genet 15, e1008155.
Ortí-Casañ, N., Zuhorn, I.S., Naudé, P.J.W., De Deyn, P.P., van Schaik, P.E.M., Wajant, H., and Eisel, U.L.M. (2022). A TNF receptor 2 agonist ameliorates neuropathology and improves cognition in an Alzheimer’s disease mouse model. Proc Natl Acad Sci USA 119, e2201137119.
Overk, C.R., and Masliah, E. (2014). Toward a unified therapeutics approach targeting putative amyloid-β oligomer receptors. Proc Natl Acad Sci USA 111, 13680–13681.
Ozmen, L., Albientz, A., Czech, C., and Jacobsen, H. (2009). Expression of transgenic APP mRNA is the key determinant for beta-amyloid deposition in PS2APP transgenic mice. Neurodegener Dis 6, 29–36.
Pang, K., Jiang, R., Zhang, W., Yang, Z., Li, L.L., Shimozawa, M., Tambaro, S., Mayer, J., Zhang, B., Li, M., et al. (2022). An App knock-in rat model for Alzheimer’s disease exhibiting Aβ and tau pathologies, neuronal death and cognitive impairments. Cell Res 32, 157–175.
Pauls, E., Bayod, S., Mateo, L., Alcalde, V., Juan-Blanco, T., Sánchez-Soto, M., Saido, T.C., Saito, T., Berrenguer-Llergo, A., Attolini, C.S.O., et al. (2021). Identification and drug-induced reversion of molecular signatures of Alzheimer’s disease onset and progression in AppNL-G-F, AppNL-F, and 3xTg-AD mouse models. Genome Med 13, 168.
Pedram, A., Razandi, M., Deschenes, R.J., and Levin, E.R. (2012). DHHC-7 and -21 are palmitoylacyltransferases for sex steroid receptors. Mol Biol Cell 23, 188–199.
Peng, Y., Sun, J., Hon, S., Nylander, A.N., Xia, W., Feng, Y., Wang, X., and Lemere, C.A. (2010). L-3-n-butylphthalide improves cognitive impairment and reduces amyloid-beta in a transgenic model of Alzheimer’s disease. J Neurosci 30, 8180–8189.
Pensalfini, A., Kim, S., Subbanna, S., Bleiwas, C., Goulbourne, C.N., Stavrides, P.H., Jiang, Y., Lee, J.H., Darji, S., Pawlik, M., et al. (2020). Endosomal dysfunction induced by directly overactivating Rab5 recapitulates prodromal and neurodegenerative features of Alzheimer’s disease. Cell Rep 33, 108420.
Perez, S.E., Raghanti, M.A., Hof, P.R., Kramer, L., Ikonomovic, M.D., Lacor, P.N., Erwin, J.M., Sherwood, C.C., and Mufson, E.J. (2013). Alzheimer’s disease pathology in the neocortex and hippocampus of the western lowland gorilla (Gorilla gorilla gorilla). J Comp Neurol 521, 4318–4338.
Peroutka, S.J. (2022). Defining demographic cohorts in clinical trial populations using large electronic health records databases. Contemp Clin Trials 121, 106890.
Petrushina, I., Hovakimyan, A., Harahap-Carrillo, I.S., Davtyan, H., Antonyan, T., Chailyan, G., Kazarian, K., Antonenko, M., Jullienne, A., Hamer, M.M., et al. (2020). Characterization and preclinical evaluation of the cGMP grade DNA based vaccine, AV-1959D to enter the first-in-human clinical trials. Neurobiol Dis 139, 104823.
Philipson, O., Hammarström, P., Nilsson, K.P.R., Portelius, E., Olofsson, T., Ingelsson, M., Hyman, B.T., Blennow, K., Lannfelt, L., Kalimo, H., et al. (2009). A highly insoluble state of Aβ similar to that of Alzheimer’s disease brain is found in Arctic APP transgenic mice. Neurobiol Aging 30, 1393–1405.
Platt, B., Drever, B., Koss, D., Stoppelkamp, S., Jyoti, A., Plano, A., Utan, A., Merrick, G., Ryan, D., Melis, V., et al. (2011). Abnormal cognition, sleep, EEG and brain metabolism in a novel knock-in Alzheimer mouse, PLB1. PLoS ONE 6, e27068.
Plucińska, K., Crouch, B., Koss, D., Robinson, L., Siebrecht, M., Riedel, G., and Platt, B. (2014). Knock-in of human BACE1 cleaves murine APP and reiterates Alzheimer-like phenotypes. J Neurosci 34, 10710–10728.
Poirier, R., Veltman, I., Pflimlin, M.C., Knoflach, F., and Metzger, F. (2010). Enhanced dentate gyrus synaptic plasticity but reduced neurogenesis in a mouse model of amyloidosis. Neurobiol Dis 40, 386–393.
Price, K.A., Varghese, M., Sowa, A., Yuk, F., Brautigam, H., Ehrlich, M.E., and Dickstein, D.L. (2014). Altered synaptic structure in the hippocampus in a mouse model of Alzheimer’s disease with soluble amyloid-β oligomers and no plaque pathology. Mol Neurodegener 9, 41.
Prüßing, K., Voigt, A., and Schulz, J.B. (2013). Drosophila melanogaster as a model organism for Alzheimer’s disease. Mol Neurodegener 8, 35.
Puig, B., Gómez-Isla, T., Ribé, E., Cuadrado, M., Torrejón-Escribano, B., Dalfó, E., and Ferrer, I. (2004). Expression of stress-activated kinases c-Jun N-terminal kinase (SAPK/JNK-P) and p38 kinase (p38-P), and tau hyperphosphorylation in neurites surrounding βA plaques in APP Tg2576 mice. Neuropathol Appl Neurobiol 30, 491–502.
Puoliväli, J., Wang, J., Heikkinen, T., Heikkilä, M., Tapiola, T., van Groen, T., and Tanila, H. (2002). Hippocampal Aβ42 levels correlate with spatial memory deficit in APP and PS1 double transgenic mice. Neurobiol Dis 9, 339–347.
Qiu, Q., Jia, L., Wang, Q., Zhao, L., Jin, H., Li, T., Quan, M., Xu, L., Li, B., Li, Y., et al. (2020). Identification of a novel PSEN1 Gly111Val missense mutation in a Chinese pedigree with early-onset Alzheimer’s disease. Neurobiol Aging 85, 155.e1–155.e4.
Qu, C., Li, Q.P., Su, Z.R., Ip, S.P., Yuan, Q.J., Xie, Y.L., Xu, Q.Q., Yang, W., Huang, Y.F., Xian, Y.F., et al. (2022). Nano-Honokiol ameliorates the cognitive deficits in TgCRND8 mice of Alzheimer’s disease via inhibiting neuropathology and modulating gut microbiota. J Adv Res 35, 231–243.
Quan, M., Zhao, T., Tang, Y., Luo, P., Wang, W., Qin, Q., Li, T., Wang, Q., Fang, J., and Jia, J. (2020). Effects of gene mutation and disease progression on representative neural circuits in familial Alzheimer’s disease. Alzheimers Res Ther 12, 14.
Radde, R., Bolmont, T., Kaeser, S.A., Coomaraswamy, J., Lindau, D., Stoltze, L., Calhoun, M.E., Jäggi, F., Wolburg, H., Gengler, S., et al. (2006). Aβ42-driven cerebral amyloidosis in transgenic mice reveals early and robust pathology. EMBO Rep 7, 940–946.
Ramsden, M., Kotilinek, L., Forster, C., Paulson, J., McGowan, E., SantaCruz, K., Guimaraes, A., Yue, M., Lewis, J., Carlson, G., et al. (2005). Age-dependent neurofibrillary tangle formation, neuron loss, and memory impairment in a mouse model of human tauopathy (P301L). J Neurosci 25, 10637–10647.
Rao, C.V., and Yamada, H.Y. (2021). How would preclinical Alzheimer’s disease (AD pathology) occur? An insight from a genomic instability mouse model. Neural Regen Res 16, 2012–2014.
Reaume, A.G., Howland, D.S., Trusko, S.P., Savage, M.J., Lang, D.M., Greenberg, B.D., Siman, R., and Scott, R.W. (1996). Enhanced amyloidogenic processing of the β-amyloid precursor protein in gene-targeted mice bearing the Swedish familial Alzheimer’s disease mutations and a “humanized” Aβ sequence. J Biol Chem 271, 23380–23388.
Reichwald, J., Danner, S., Wiederhold, K.H., and Staufenbiel, M. (2009). Expression of complement system components during aging and amyloid deposition in APP transgenic mice. J Neuroinflammation 6, 35.
Richard, B.C., Kurdakova, A., Baches, S., Bayer, T.A., Weggen, S., and Wirths, O. (2015). Gene dosage dependent aggravation of the neurological phenotype in the 5XFAD mouse model of Alzheimer’s disease. J Alzheimer Dis 45, 1223–1236.
Richards, J.G., Higgins, G.A., Ouagazzal, A.M., Ozmen, L., Kew, J.N.C., Bohrmann, B., Malherbe, P., Brockhaus, M., Loetscher, H., Czech, C., et al. (2003). PS2APP transgenic mice, coexpressing hPS2mut and hAPPswe, show age-related cognitive deficits associated with discrete brain amyloid deposition and inflammation. J Neurosci 23, 8989–9003.
Richardson, J.C., Kendal, C.E., Anderson, R., Priest, F., Gower, E., Soden, P., Gray, R., Topps, S., Howlett, D.R., Lavender, D., et al. (2003). Ultrastructural and behavioural changes precede amyloid deposition in a transgenic model of Alzheimer’s disease. Neuroscience 122, 213–228.
Rockenstein, E., Mallory, M., Mante, M., Sisk, A., and Masliaha, E. (2001). Early formation of mature amyloid-beta protein deposits in a mutant APP transgenic model depends on levels of Aβ1–42. J Neurosci Res 66, 573–582.
Rockenstein, E., Mante, M., Alford, M., Adame, A., Crews, L., Hashimoto, M., Esposito, L., Mucke, L., and Masliah, E. (2005). High β-secretase activity elicits neurodegeneration in transgenic mice despite reductions in amyloid-β levels. J Biol Chem 280, 32957–32967.
Roder, S., Danober, L., Pozza, M.F., Lingenhoehl, K., Wiederhold, K.H., and Olpe, H.R. (2003). Electrophysiological studies on the hippocampus and prefrontal cortex assessing the effects of amyloidosis in amyloid precursor protein 23 transgenic mice. Neuroscience 120, 705–720.
Rönnbäck, A., Sagelius, H., Bergstedt, K.D., Näslund, J., Westermark, G. T., Winblad, B., and Graff, C. (2012). Amyloid neuropathology in the single Arctic APP transgenic model affects interconnected brain regions. Neurobiol Aging 33, 831.e11–831.e19.
Rupp, N.J., Wegenast-Braun, B.M., Radde, R., Calhoun, M.E., and Jucker, M. (2011). Early onset amyloid lesions lead to severe neuritic abnormalities and local, but not global neuron loss in APPPS1 transgenic mice. Neurobiol Aging 32, 2324.e1–2324.e6.
Sadowski, M., Pankiewicz, J., Scholtzova, H., Ji, Y., Quartermain, D., Jensen, C.H., Duff, K., Nixon, R.A., Gruen, R.J., and Wisniewski, T. (2004). Amyloid-β deposition is associated with decreased hippocampal glucose metabolism and spatial memory impairment in APP/PS1 mice. J Neuropathol Exp Neurol 63, 418–428.
Saganich, M.J., Schroeder, B.E., Galvan, V., Bredesen, D.E., Koo, E.H., and Heinemann, S.F. (2006). Deficits in synaptic transmission and learning in amyloid precursor protein (APP) transgenic mice require C-terminal cleavage of APP. J Neurosci 26, 13428–13436.
Sagare, A.P., Bell, R.D., Zhao, Z., Ma, Q., Winkler, E.A., Ramanathan, A., and Zlokovic, B.V. (2013). Pericyte loss influences Alzheimer-like neurodegeneration in mice. Nat Commun 4, 2932.
Saito, S., Yamamoto, Y., Maki, T., Hattori, Y., Ito, H., Mizuno, K., Harada-Shiba, M., Kalaria, R.N., Fukushima, M., Takahashi, R., et al. (2017). Taxifolin inhibits amyloid-β oligomer formation and fully restores vascular integrity and memory in cerebral amyloid angiopathy. Acta Neuropathol Commun 5, 26.
Saito, T., Matsuba, Y., Mihira, N., Takano, J., Nilsson, P., Itohara, S., Iwata, N., and Saido, T.C. (2014). Single App knock-in mouse models of Alzheimer’s disease. Nat Neurosci 17, 661–663.
Saito, T., Mihira, N., Matsuba, Y., Sasaguri, H., Hashimoto, S., Narasimhan, S., Zhang, B., Murayama, S., Higuchi, M., Lee, V.M.Y., et al. (2019). Humanization of the entire murine Mapt gene provides a murine model of pathological human tau propagation. J Biol Chem 294, 12754–12765.
Saito, T., Suemoto, T., Brouwers, N., Sleegers, K., Funamoto, S., Mihira, N., Matsuba, Y., Yamada, K., Nilsson, P., Takano, J., et al. (2011). Potent amyloidogenicity and pathogenicity of Aβ43. Nat Neurosci 14, 1023–1032.
Sanchez-Varo, R., Mejias-Ortega, M., Fernandez-Valenzuela, J.J., Nuñez-Diaz, C., Caceres-Palomo, L., Vegas-Gomez, L., Sanchez-Mejias, E., Trujillo-Estrada, L., Garcia-Leon, J.A., Moreno-Gonzalez, I., et al. (2022). Transgenic mouse models of Alzheimer’s disease: an integrative analysis. Int J Mol Sci 23, 5404.
SantaCruz, K., Lewis, J., Spires, T., Paulson, J., Kotilinek, L., Ingelsson, M., Guimaraes, A., DeTure, M., Ramsden, M., McGowan, E., et al. (2005). Tau suppression in a neurodegenerative mouse model improves memory function. Science 309, 476–481.
Sasaguri, H., Hashimoto, S., Watamura, N., Sato, K., Takamura, R., Nagata, K., Tsubuki, S., Ohshima, T., Yoshiki, A., Sato, K., et al. (2022). Recent advances in the modeling of Alzheimer’s disease. Front Neurosci 16, 807473.
Sasaguri, H., Nilsson, P., Hashimoto, S., Nagata, K., Saito, T., De Strooper, B., Hardy, J., Vassar, R., Winblad, B., and Saido, T.C. (2017). APP mouse models for Alzheimer’s disease preclinical studies. EMBO J 36, 2473–2487.
Sato, K., Watamura, N., Fujioka, R., Mihira, N., Sekiguchi, M., Nagata, K., Ohshima, T., Saito, T., Saido, T.C., and Sasaguri, H. (2021). A third-generation mouse model of Alzheimer’s disease shows early and increased cored plaque pathology composed of wild-type human amyloid β peptide. J Biol Chem 297, 101004.
Savonenko, A., Xu, G.M., Melnikova, T., Morton, J.L., Gonzales, V., Wong, M.P.F., Price, D.L., Tang, F., Markowska, A.L., and Borchelt, D. R. (2005). Episodic-like memory deficits in the APPswe/PS1dE9 mouse model of Alzheimer’s disease: Relationships to β-amyloid deposition and neurotransmitter abnormalities. Neurobiol Dis 18, 602–617.
Savonenko, A.V., Xu, G.M., Price, D.L., Borchelt, D.R., and Markowska, A.L. (2003). Normal cognitive behavior in two distinct congenic lines of transgenic mice hyperexpressing mutant APPSWE. Neurobiol Dis 12, 194–211.
Scattoni, M.L., Gasparini, L., Alleva, E., Goedert, M., Calamandrei, G., and Spillantini, M.G. (2010). Early behavioural markers of disease in P301S tau transgenic mice. Behav Brain Res 208, 250–257.
Scearce-Levie, K., Sanchez, P.E., and Lewcock, J.W. (2020). Leveraging preclinical models for the development of Alzheimer disease therapeutics. Nat Rev Drug Discov 19, 447–462.
Schneider, I., Reversé, D., Dewachter, I., Ris, L., Caluwaerts, N., Kuipéri, C., Gilis, M., Geerts, H., Kretzschmar, H., Godaux, E., et al. (2001). Mutant presenilins disturb neuronal calcium homeostasis in the brain of transgenic mice, decreasing the threshold for excitotoxicity and facilitating long-term potentiation. J Biol Chem 276, 11539–11544.
Sebollela, A., Cline, E.N., Popova, I., Luo, K., Sun, X., Ahn, J., Barcelos, M.A., Bezerra, V.N., Lyra e Silva, N.M., Patel, J., et al. (2017). A human scFv antibody that targets and neutralizes high molecular weight pathogenic amyloid-β oligomers. J Neurochem 142, 934–947.
Sehar, U., Rawat, P., Reddy, A.P., Kopel, J., and Reddy, P.H. (2022). Amyloid beta in aging and Alzheimer’s disease. Int J Mol Sci 23, 12924.
Selkoe, D.J., and Hardy, J. (2016). The amyloid hypothesis of Alzheimer’s disease at 25 years. EMBO Mol Med 8, 595–608.
Serneels, L., Van Biervliet, J., Craessaerts, K., Dejaegere, T., Horré, K., Van Houtvin, T., Esselmann, H., Paul, S., Schäfer, M.K., Berezovska, O., et al. (2009). γ-Secretase heterogeneity in the Aph1 subunit: relevance for Alzheimer’s disease. Science 324, 639–642.
Sevigny, J., Chiao, P., Bussière, T., Weinreb, P.H., Williams, L., Maier, M., Dunstan, R., Salloway, S., Chen, T., Ling, Y., et al. (2016). The antibody aducanumab reduces Aβ plaques in Alzheimer’s disease. Nature 537, 50–56.
Shen, B., Zhang, J., Wu, H., Wang, J., Ma, K., Li, Z., Zhang, X., Zhang, P., and Huang, X. (2013). Generation of gene-modified mice via Cas9/RNA-mediated gene targeting. Cell Res 23, 720–723.
Shen, L., Qin, W., Wu, L., Zhou, A., Tang, Y., Wang, Q., Jia, L., and Jia, J. (2019). Two novel presenilin-1 mutations (I249L and P433S) in early onset Chinese Alzheimer’s pedigrees and their functional characterization. Biochem Biophys Res Commun 516, 264–269.
Shimojo, M., Sahara, N., Mizoroki, T., Funamoto, S., Morishima-Kawashima, M., Kudo, T., Takeda, M., Ihara, Y., Ichinose, H., and Takashima, A. (2008). Enzymatic characteristics of I213T mutant presenilin-1/γ-secretase in cell models and knock-in mouse brains. J Biol Chem 283, 16488–16496.
Sierksma, A., Escott-Price, V., and De Strooper, B. (2020). Translating genetic risk of Alzheimer’s disease into mechanistic insight and drug targets. Science 370, 61–66.
Skaaraas, G.H.E.S., Melbye, C., Puchades, M.A., Leung, D.S.Y., Jacobsen, Ø., Rao, S.B., Ottersen, O.P., Leergaard, T.B., and Torp, R. (2021). Cerebral amyloid angiopathy in a mouse model of Alzheimer’s disease associates with upregulated angiopoietin and downregulated hypoxia-inducible factor. J Alzheimer Dis 83, 1651–1663.
Snellman, A., López-Picón, F.R., Rokka, J., Salmona, M., Forloni, G., Scheinin, M., Solin, O., Rinne, J.O., and Haaparanta-Solin, M. (2013). Longitudinal amyloid imaging in mouse brain with 11C-PIB: comparison of APP23, Tg2576, and APPswe-PS1dE9 mouse models of Alzheimer disease. J Nucl Med 54, 1434–1441.
Song, W.M., Joshita, S., Zhou, Y., Ulland, T.K., Gilfillan, S., and Colonna, M. (2018). Humanized TREM2 mice reveal microglia-intrinsic and -extrinsic effects of R47H polymorphism. J Exp Med 215, 745–760.
Soriano, P. (1999). Generalized lacZ expression with the ROSA26 Cre reporter strain. Nat Genet 21, 70–71.
Spires, T.L., Orne, J.D., SantaCruz, K., Pitstick, R., Carlson, G.A., Ashe, K. H., and Hyman, B.T. (2006). Region-specific dissociation of neuronal loss and neurofibrillary pathology in a mouse model of tauopathy. Am J Pathol 168, 1598–1607.
Stalder, M., Phinney, A., Probst, A., Sommer, B., Staufenbiel, M., and Jucker, M. (1999). Association of microglia with amyloid plaques in brains of APP23 transgenic mice. Am J Pathol 154, 1673–1684.
Stover, K.R., Campbell, M.A., Van Winssen, C.M., and Brown, R.E. (2015). Early detection of cognitive deficits in the 3xTg-AD mouse model of Alzheimer’s disease. Behav Brain Res 289, 29–38.
Sturchler-Pierrat, C., Abramowski, D., Duke, M., Wiederhold, K.H., Mistl, C., Rothacher, S., Ledermann, B., Bürki, K., Frey, P., Paganetti, P.A., et al. (1997). Two amyloid precursor protein transgenic mouse models with Alzheimer disease-like pathology. Proc Natl Acad Sci USA 94, 13287–13292.
Sun, B., Chen, Y., Fan, D., Zhu, C., Zeng, F., and Wang, Y. (2021). Critical thinking on amyloid-beta-targeted therapy: challenges and perspectives. Sci China Life Sci 64, 926–937.
Sun, Z.Q., Liu, J.F., Luo, W., Wong, C.H., So, K.F., Hu, Y., and Chiu, K. (2022). Lycium barbarum extract promotes M2 polarization and reduces oligomeric amyloid-β-induced inflammatory reactions in microglial cells. Neural Regen Res 17, 203–209.
Swanson, C.J., Zhang, Y., Dhadda, S., Wang, J., Kaplow, J., Lai, R.Y.K., Lannfelt, L., Bradley, H., Rabe, M., Koyama, A., et al. (2021). A randomized, double-blind, phase 2b proof-of-concept clinical trial in early Alzheimer’s disease with lecanemab, an anti-Aβ protofibril antibody. Alzheimers Res Ther 13, 80.
Sydow, A., Hochgräfe, K., Könen, S., Cadinu, D., Matenia, D., Petrova, O., Joseph, M., Dennissen, F.J., and Mandelkow, E.M. (2016). Age-dependent neuroinflammation and cognitive decline in a novel Ala152Thr-Tau transgenic mouse model of PSP and AD. Acta Neuropathol Commun 4, 17.
Sydow, A., Van der Jeugd, A., Zheng, F., Ahmed, T., Balschun, D., Petrova, O., Drexler, D., Zhou, L., Rune, G., Mandelkow, E., et al. (2011). Tau-induced defects in synaptic plasticity, learning, and memory are reversible in transgenic mice after switching off the toxic Tau mutant. J Neurosci 31, 2511–2525.
Takahashi, R.H., Almeida, C.G., Kearney, P.F., Yu, F., Lin, M.T., Milner, T. A., and Gouras, G.K. (2004). Oligomerization of Alzheimer’s β-amyloid within processes and synapses of cultured neurons and brain. J Neurosci 24, 3592–3599.
Takeuchi, H., Iba, M., Inoue, H., Higuchi, M., Takao, K., Tsukita, K., Karatsu, Y., Iwamoto, Y., Miyakawa, T., Suhara, T., et al. (2011). P301S mutant human tau transgenic mice manifest early symptoms of human tauopathies with dementia and altered sensorimotor gating. PLoS ONE 6, e21050.
Tanemura, K., Akagi, T., Murayama, M., Kikuchi, N., Murayama, O., Hashikawa, T., Yoshiike, Y., Park, J.M., Matsuda, K., Nakao, S., et al. (2001). Formation of filamentous tau aggregations in transgenic mice expressing V337M human tau. Neurobiol Dis 8, 1036–1045.
Tanemura, K., Chui, D.H., Fukuda, T., Murayama, M., Park, J.M., Akagi, T., Tatebayashi, Y., Miyasaka, T., Kimura, T., Hashikawa, T., et al. (2006). Formation of tau inclusions in knock-in mice with familial Alzheimer disease (FAD) mutation of presenilin 1 (PS1). J Biol Chem 281, 5037–5041.
Tanemura, K., Murayama, M., Akagi, T., Hashikawa, T., Tominaga, T., Ichikawa, M., Yamaguchi, H., and Takashima, A. (2002). Neurodegeneration with tau accumulation in a transgenic mouse expressing V337M human tau. J Neurosci 22, 133–141.
Tatebayashi, Y., Miyasaka, T., Chui, D.H., Akagi, T., Mishima, K., Iwasaki, K., Fujiwara, M., Tanemura, K., Murayama, M., Ishiguro, K., et al. (2002). Tau filament formation and associative memory deficit in aged mice expressing mutant (R406W) human tau. Proc Natl Acad Sci USA 99, 13896–13901.
Terwel, D., Lasrado, R., Snauwaert, J., Vandeweert, E., Van Haesendonck, C., Borghgraef, P., and Van Leuven, F. (2005). Changed conformation of mutant Tau-P301L underlies the moribund tauopathy, absent in progressive, nonlethal axonopathy of Tau-4R/2N transgenic mice. J Biol Chem 280, 3963–3973.
Thomas, K.R., and Capecchi, M.R. (1987). Site-directed mutagenesis by gene targeting in mouse embryo-derived stem cells. Cell 51, 503–512.
Tian, Y., Wang, W., Xu, L., Li, H., Wei, Y., Wu, Q., and Jia, J. (2019). Activation of Nrf2/ARE pathway alleviates the cognitive deficits in PS1V97L-Tg mouse model of Alzheimer’s disease through modulation of oxidative stress. J Neurosci Res 97, 492–505.
Tomiyama, T., Matsuyama, S., Iso, H., Umeda, T., Takuma, H., Ohnishi, K., Ishibashi, K., Teraoka, R., Sakama, N., Yamashita, T., et al. (2010). A mouse model of amyloid β oligomers: their contribution to synaptic alteration, abnormal tau phosphorylation, glial activation, and neuronal loss in vivo. J Neurosci 30, 4845–4856.
Tomiyama, T., Nagata, T., Shimada, H., Teraoka, R., Fukushima, A., Kanemitsu, H., Takuma, H., Kuwano, R., Imagawa, M., Ataka, S., et al. (2008). A new amyloid β variant favoring oligomerization in Alzheimer’s-type dementia. Ann Neurol 63, 377–387.
Tomiyama, T., and Shimada, H. (2020). APP Osaka mutation in familial Alzheimer’s disease—its discovery, phenotypes, and mechanism of recessive inheritance. Int J Mol Sci 21, 1413.
Tucker, S., Möller, C., Tegerstedt, K., Lord, A., Laudon, H., Sjödahl, J., Söderberg, L., Spens, E., Sahlin, C., Waara, E.R., et al. (2015). The murine version of BAN2401 (mAb158) selectively reduces amyloid-β protofibrils in brain and cerebrospinal fluid of tg-ArcSwe mice. J Alzheimer Dis 43, 575–588.
Umeda, T., Kimura, T., Yoshida, K., Takao, K., Fujita, Y., Matsuyama, S., Sakai, A., Yamashita, M., Yamashita, Y., Ohnishi, K., et al. (2017). Mutation-induced loss of APP function causes GABAergic depletion in recessive familial Alzheimer’s disease: analysis of Osaka mutation-knockin mice. Acta Neuropathol Commun 5, 59.
Umeda, T., Maekawa, S., Kimura, T., Takashima, A., Tomiyama, T., and Mori, H. (2014). Neurofibrillary tangle formation by introducing wild-type human tau into APP transgenic mice. Acta Neuropathol 127, 685–698.
Umeda, T., Tomiyama, T., Sakama, N., Tanaka, S., Lambert, M.P., Klein, W.L., and Mori, H. (2011). Intraneuronal amyloid β oligomers cause cell death via endoplasmic reticulum stress, endosomal/lysosomal leakage, and mitochondrial dysfunction in vivo. J Neurosci Res 89, 1031–1042.
Van Dam, D., D’Hooge, R., Staufenbiel, M., Van Ginneken, C., Van Meir, F., and De Deyn, P.P. (2003). Age-dependent cognitive decline in the APP23 model precedes amyloid deposition. Eur J Neurosci 17, 388–396.
Van der Jeugd, A., Hochgräfe, K., Ahmed, T., Decker, J.M., Sydow, A., Hofmann, A., Wu, D., Messing, L., Balschun, D., D’Hooge, R., et al. (2012). Cognitive defects are reversible in inducible mice expressing pro-aggregant full-length human Tau. Acta Neuropathol 123, 787–805.
Van Dorpe, J., Smeijers, L., Dewachter, I., Nuyens, D., Spittaels, K., Van den Haute, C., Mercken, M., Moechars, D., Laenen, I., Kuiperi, C., et al. (2000). Prominent cerebral amyloid angiopathy in transgenic mice overexpressing the london mutant of human APP in neurons. Am J Pathol 157, 1283–1298.
van Dyck, C.H., Swanson, C.J., Aisen, P., Bateman, R.J., Chen, C., Gee, M., Kanekiyo, M., Li, D., Reyderman, L., Cohen, S., et al. (2023). Lecanemab in early Alzheimer’s disease. N Engl J Med 388, 9–21.
van Ham, T.J., Breitling, R., Swertz, M.A., and Nollen, E.A.A. (2009). Neurodegenerative diseases: Lessons from genome-wide screens in small model organisms. EMBO Mol Med 1, 360–370.
Van Skike, C.E., Hussong, S.A., Hernandez, S.F., Banh, A.Q., DeRosa, N., and Galvan, V. (2021). mTOR attenuation with rapamycin reverses neurovascular uncoupling and memory deficits in mice modeling Alzheimer’s disease. J Neurosci 41, 4305–4320.
Veitch, D.P., Weiner, M.W., Aisen, P.S., Beckett, L.A., Cairns, N.J., Green, R.C., Harvey, D., Jack Jr., C.R., Jagust, W., Morris, J.C., et al. (2019). Understanding disease progression and improving Alzheimer’s disease clinical trials: recent highlights from the Alzheimer’s Disease Neuroimaging Initiative. Alzheimers Dement 15, 106–152.
Vitale, F., Ortolan, J., Volpe, B.T., Marambaud, P., Giliberto, L., and d’Abramo, C. (2020). Intramuscular injection of vectorized-scFvMC1 reduces pathological tau in two different tau transgenic models. Acta Neuropathol Commun 8, 126.
Volianskis, A., Køstner, R., Mølgaard, M., Hass, S., and Jensen, M.S. (2010). Episodic memory deficits are not related to altered glutamatergic synaptic transmission and plasticity in the CA1 hippocampus of the APPswe/PS1ΔE9-deleted transgenic mice model of β-amyloidosis. Neurobiol Aging 31, 1173–1187.
Wang, H., Xu, X.X., Pan, Y.C., Yan, Y.X., Hu, X.Y., Chen, R.W., Ravoo, B. J., Guo, D.S., and Zhang, T. (2021a). Recognition and removal of amyloid-β by a heteromultivalent macrocyclic coassembly: a potential strategy for the treatment of Alzheimer’s disease. Adv Mater 33, 2006483.
Wang, J., Guo, X., Lu, W., Liu, J., Zhang, H., Quan, Q., Su, H., Ma, L., Gao, F., and Qu, Q. (2021b). Donepezil combined with DL-3-n-butylphthalide delays cognitive decline in patients with mild to moderate Alzheimer’s disease: a multicenter, prospective cohort study. J Alzheimer Dis 80, 673–681.
Wang, J., Tanila, H., Puoliväli, J., Kadish, I., and Groen, T. (2003). Gender differences in the amount and deposition of amyloidβ in APPswe and PS1 double transgenic mice. Neurobiol Dis 14, 318–327.
Wang, R., Dineley, K.T., Sweatt, J.D., and Zheng, H. (2004). Presenilin 1 familial Alzheimer’s disease mutation leads to defective associative learning and impaired adult neurogenesis. Neuroscience 126, 305–312.
Wang, W., Lu, L., Wu, Q., and Jia, J. (2016). Brain amyloid-β plays an initiating role in the pathophysiological process of the PS1V97L-Tg mouse model of Alzheimer’s disease. J Alzheimer Dis 52, 1089–1099.
Wang, W., Wei, C., Quan, M., Li, T., and Jia, J. (2020). Sulforaphane reverses the amyloid-β oligomers induced depressive-like behavior. J Alzheimer Dis 78, 127–137.
Wang, W.Z., Li, M.W., Chen, Y., Liu, L.Y., Xu, Y., Xia, Z.H., Yu, Y., Wang, X.D., Chen, W., Zhang, F., et al. (2021c). 3xTg-AD mice overexpressing phospholipid transfer protein improves cognition through decreasing amyloid-beta production and tau hyperphosphorylation. J Alzheimers Dis 82, 1635–1649.
Wang, X., Sun, G., Feng, T., Zhang, J., Huang, X., Wang, T., Xie, Z., Chu, X., Yang, J., Wang, H., et al. (2019). Sodium oligomannate therapeutically remodels gut microbiota and suppresses gut bacterial amino acids-shaped neuroinflammation to inhibit Alzheimer’s disease progression. Cell Res 29, 787–803.
Wang, Y., Cella, M., Mallinson, K., Ulrich, J.D., Young, K.L., Robinette, M.L., Gilfillan, S., Krishnan, G.M., Sudhakar, S., Zinselmeyer, B.H., et al. (2015). TREM2 lipid sensing sustains the microglial response in an Alzheimer’s disease model. Cell 160, 1061–1071.
Wang, Y., Cheng, Z., Qin, W., and Jia, J. (2012). Val97Leu mutant presenilin-1 induces tau hyperphosphorylation and spatial memory deficit in mice and the underlying mechanisms. J Neurochem 121, 135–145.
Watamura, N., Sato, K., and Saido, T.C. (2022a). Mouse models of Alzheimer’s disease for preclinical research. Neurochem Int 158, 105361.
Watamura, N., Sato, K., Shiihashi, G., Iwasaki, A., Kamano, N., Takahashi, M., Sekiguchi, M., Mihira, N., Fujioka, R., Nagata, K., et al. (2022b). An isogenic panel of App knock-in mouse models: Profiling β-secretase inhibition and endosomal abnormalities. Sci Adv 8, eabm6155.
Weidensteiner, C., Metzger, F., Bohrmann, A.B.B., Kuennecke, B., and von Kienlin, M. (2009). Cortical hypoperfusion in the B6.PS2APP mouse model for Alzheimer’s disease: Comprehensive phenotyping of vascular and tissular parameters by MRI. Magn Reson Med 62, 35–45.
Wen, P.H., Hof, P.R., Chen, X., Gluck, K., Austin, G., Younkin, S.G., Younkin, L.H., DeGasperi, R., Gama Sosa, M.A., Robakis, N.K., et al. (2004). The presenilin-1 familial Alzheimer disease mutant P117L impairs neurogenesis in the hippocampus of adult mice. Exp Neurol 188, 224–237.
Wen, P.H., Shao, X., Shao, Z., Hof, P.R., Wisniewski, T., Kelley, K., Friedrich Jr., V.L., Ho, L., Pasinetti, G.M., Shioi, J., et al. (2002). Overexpression of wild type but not an FAD mutant presenilin-1 promotes neurogenesis in the hippocampus of adult mice. Neurobiol Dis 10, 8–19.
Wilcock, D.M., Lewis, M.R., Van Nostrand, W.E., Davis, J., Previti, M.L., Gharkholonarehe, N., Vitek, M.P., and Colton, C.A. (2008). Progression of amyloid pathology to Alzheimer’s disease pathology in an amyloid precursor protein transgenic mouse model by removal of nitric oxide synthase 2. J Neurosci 28, 1537–1545.
Wilhelmus, M.M.M., Chouchane, O., Loos, M., Jongenelen, C.A.M., Brevé, J.J.P., Jonker, A., Bol, J.G.J.M., Smit, A.B., and Drukarch, B. (2022). Absence of tissue transglutaminase reduces amyloid-beta pathology in APP23 mice. Neuropathol Appl Neurobio 48, e12796.
Williams, T., Ruiz, A.J., Ruiz, A.M., Vo, Q., Tsering, W., Xu, G., McFarland, K., Giasson, B.I., Sullivan, P., Borchelt, D.R., et al. (2022). Impact of APOE genotype on prion-type propagation of tauopathy. Acta Neuropathol Commun 10, 57.
Willuweit, A., Velden, J., Godemann, R., Manook, A., Jetzek, F., Tintrup, H., Kauselmann, G., Zevnik, B., Henriksen, G., Drzezga, A., et al. (2009). Early-onset and robust amyloid pathology in a new homozygous mouse model of Alzheimer’s disease. PLoS ONE 4, e7931.
Wirths, O., Erck, C., Martens, H., Harmeier, A., Geumann, C., Jawhar, S., Kumar, S., Multhaup, G., Walter, J., Ingelsson, M., et al. (2010). Identification of low molecular weight pyroglutamate Aβ oligomers in Alzheimer disease. J Biol Chem 285, 41517–41524.
Wittnam, J.L., Portelius, E., Zetterberg, H., Gustavsson, M.K., Schilling, S., Koch, B., Demuth, H.U., Blennow, K., Wirths, O., and Bayer, T.A. (2012). Pyroglutamate amyloid β (Aβ) aggravates behavioral deficits in transgenic amyloid mouse model for Alzheimer disease. J Biol Chem 287, 8154–8162.
Wong, T.H., Seelaar, H., Melhem, S., Rozemuller, A.J.M., and van Swieten, J.C. (2020). Genetic screening in early-onset Alzheimer’s disease identified three novel presenilin mutations. Neurobiol Aging 86, 201. e9–201.e14.
Wright, A.L., Zinn, R., Hohensinn, B., Konen, L.M., Beynon, S.B., Tan, R. P., Clark, I.A., Abdipranoto, A., and Vissel, B. (2013). Neuroinflammation and neuronal loss precede Aβ plaque deposition in the hAPP-J20 mouse model of Alzheimer’s disease. PLoS ONE 8, e59586.
Xia, D., Lianoglou, S., Sandmann, T., Calvert, M., Suh, J., Thomsen, E., Dugas, J., Pizzo, M., Devos, S., Earr, T., et al. (2021). Fibrillar Aβ causes profound microglial metabolic perturbations in a novel APP knock-in mouse model. BioRxiv, 2021.01.19.426731.
Xian, Y.F., Qu, C., Liu, Y., Ip, S.P., Yuan, Q.J., Yang, W., and Lin, Z.X. (2020). Magnolol ameliorates behavioral impairments and neuropathology in a transgenic mouse model of Alzheimer’s disease. Oxid Med Cell Longev 2020, 1–17.
Xiao, N.A., Zhang, J., Zhou, M., Wei, Z., Wu, X.L., Dai, X.M., Zhu, Y.G., and Chen, X.C. (2015). Reduction of glucose metabolism in olfactory bulb is an earlier Alzheimer’s disease-related biomarker in 5XFAD mice. Chin Med J 128, 2220–2227.
Xie, Z., Meng, J., Kong, W., Wu, Z., Lan, F., Narengaowa, F., Hayashi, Y., Yang, Q., Bai, Z., Nakanishi, H., et al. (2022). Microglial cathepsin E plays a role in neuroinflammation and amyloid β production in Alzheimer’s disease. Aging Cell 21, e13565.
Xu, F., Grande, A.M., Robinson, J.K., Previti, M.L., Vasek, M., Davis, J., and Van Nostrand, W.E. (2007). Early-onset subicular microvascular amyloid and neuroinflammation correlate with behavioral deficits in vasculotropic mutant amyloid β-protein precursor transgenic mice. Neuroscience 146, 98–107.
Xu, H., Rösler, T.W., Carlsson, T., de Andrade, A., Bruch, J., Höllerhage, M., Oertel, W.H., and Höglinger, G.U. (2014). Memory deficits correlate with tau and spine pathology in P301S MAPT transgenic mice. Neuropathol Appl Neurobiol 40, 833–843.
Yamada, K., Yabuki, C., Seubert, P., Schenk, D., Hori, Y., Ohtsuki, S., Terasaki, T., Hashimoto, T., and Iwatsubo, T. (2009). Aβ immunotherapy: intracerebral sequestration of Aβ by an anti-Aβ monoclonal antibody 266 with high affinity to soluble Aβ. J Neurosci 29, 11393–11398.
Yao, Y., Ren, Z., Yang, R., Mei, Y., Dai, Y., Cheng, Q., Xu, C., Xu, X., Wang, S., Kim, K.M., et al. (2022). Salidroside reduces neuropathology in Alzheimer’s disease models by targeting NRF2/SIRT3 pathway. Cell Biosci 12, 180.
Yi-Bin, W., Xiang, L., Bing, Y., Qi, Z., Fei-Tong, J., Minghong, W., Xiangxiang, Z., Le, K., Yan, L., Ping, S., et al. (2022). Inhibition of the CEBPβ-NFκB interaction by nanocarrier-packaged Carnosic acid ameliorates glia-mediated neuroinflammation and improves cognitive function in an Alzheimer’s disease model. Cell Death Dis 13, 318.
Yoshiyama, Y., Higuchi, M., Zhang, B., Huang, S.M., Iwata, N., Saido, T. C., Maeda, J., Suhara, T., Trojanowski, J.Q., and Lee, V.M.Y. (2007). Synapse loss and microglial activation precede tangles in a P301S tauopathy mouse model. Neuron 53, 337–351.
Youmans, K.L., Tai, L.M., Nwabuisi-Heath, E., Jungbauer, L., Kanekiyo, T., Gan, M., Kim, J., Eimer, W.A., Estus, S., Rebeck, G.W., et al. (2012). APOE4-specific changes in Aβ accumulation in a new transgenic mouse model of Alzheimer disease. J Biol Chem 287, 41774–41786.
Yu, J.T., Xu, W., Tan, C.C., Andrieu, S., Suckling, J., Evangelou, E., Pan, A., Zhang, C., Jia, J., Feng, L., et al. (2020). Evidence-based prevention of Alzheimer’s disease: systematic review and meta-analysis of 243 observational prospective studies and 153 randomised controlled trials. J Neurol Neurosurg Psychiatry 91, 1201–1209.
Yue, M., Hanna, A., Wilson, J., Roder, H., and Janus, C. (2011). Sex difference in pathology and memory decline in rTg4510 mouse model of tauopathy. Neurobiol Aging 32, 590–603.
Zampar, S., and Wirths, O. (2021). Characterization of a mouse model of Alzheimer’s disease expressing Aβ4–42 and human mutant tau. Int J Mol Sci 22, 5191.
Zhang, H., Han, T., Zhang, L., Yu, C.H., Wan, D.G., Rahman, K., Qin, L.P., and Peng, C. (2008). Effects of tenuifolin extracted from radix polygalae on learning and memory: a behavioral and biochemical study on aged and amnesic mice. Phytomedicine 15, 587–594.
Zhang, L., Chen, C., Mak, M.S., Lu, J., Wu, Z., Chen, Q., Han, Y., Li, Y., and Pi, R. (2020). Advance of sporadic Alzheimer’s disease animal models. Med Res Rev 40, 431–458.
Zhang, X., Wei, X., Mei, Y., Wang, D., Wang, J., Zhang, Y., Li, X., Gu, Y., Peng, G., and Sun, B. (2021). Modulating adult neurogenesis affects synaptic plasticity and cognitive functions in mouse models of Alzheimer’s disease. Stem Cell Rep 16, 3005–3019.
Zhang, Y., Huang, L.J., Shi, S., Xu, S.F., Wang, X.L., and Peng, Y. (2016). L-3-n-butylphthalide rescues hippocampal synaptic failure and attenuates neuropathology in aged APP/PS1 mouse model of Alzheimer’s disease. CNS Neurosci Ther 22, 979–987.
Zhang, Y., Lu, L., Jia, J., Jia, L., Geula, C., Pei, J., Xu, Z., Qin, W., Liu, R., Li, D., et al. (2014). A lifespan observation of a novel mouse model: in vivo evidence supports Aβ oligomer hypothesis. PLoS ONE 9, e85885.
Zhao, S., Fan, Z., Zhang, X., Li, Z., Shen, T., Li, K., Yan, Y., Yuan, Y., Pu, J., Tian, J., et al. (2023). Metformin attenuates tau pathology in tau-seeded PS19 mice. Neurotherapeutics 20, 452–463.
Zhong, W., Wu, A., Berglund, K., Gu, X., Jiang, M.Q., Talati, J., Zhao, J., Wei, L., and Yu, S.P. (2022). Pathogenesis of sporadic Alzheimer’s disease by deficiency of NMDA receptor subunit GluN3A. Alzheimers Dement 18, 222–239.
Zou, C., Montagna, E., Shi, Y., Peters, F., Blazquez-Llorca, L., Shi, S., Filser, S., Dorostkar, M.M., and Herms, J. (2015). Intraneuronal APP and extracellular Aβ independently cause dendritic spine pathology in transgenic mouse models of Alzheimer’s disease. Acta Neuropathol 129, 909–920.
Zufferey, V., Vallet, P.G., Moeri, M., Moulin-Sallanon, M., Piotton, F., Marin, P., and Savioz, A. (2013). Maladaptive exploratory behavior and neuropathology of the PS-1 P117L Alzheimer transgenic mice. Brain Res Bull 94, 17–22.
Acknowledgements
This work was supported by the National Natural Science Foundation of China (U20A20354, 81530036), Beijing Brain Initiative from Beijing Municipal Science & Technology Commission (Z201100005520016, Z201100005520017), the National Major R&D Projects of China-Scientific Technological Innovation 2030 (2021ZD0201802), the National Key Scientific Instrument and Equipment Development Project (31627803), Youth Program of National Natural Science Foundation of China (81801048, 82101503) and Youth Elite Scientists Sponsorship Program by CAST (YESS20200155). We would like to thank Shuya Nie, Fangyu Li, Bingqiu Li and Wenying Liu for literature search for the clinical translation related sections.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Compliance and ethics The author(s) declare that they have no conflict of interest.
Rights and permissions
About this article
Cite this article
Li, X., Quan, M., Wei, Y. et al. Critical thinking of Alzheimer’s transgenic mouse model: current research and future perspective. Sci. China Life Sci. 66, 2711–2754 (2023). https://doi.org/10.1007/s11427-022-2357-x
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11427-022-2357-x