Abstract
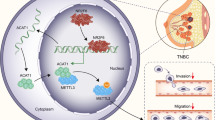
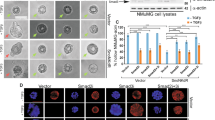
Slug, a member of the Snail family of transcriptional repressors, plays a key role in cancer progression, cellular plasticity, and epithelial to mesenchymal transition (EMT). Slug is a fast-turnover protein and its stability is controlled by post-translational modifications. Here, we identified that Slug is acetylated by acetyltransferase CREB-binding protein (CBP) in breast cancer cells. CBP directly interacts with the C-terminal domain of Slug through its catalytic histone acetyltransferase (HAT) domain, leading to acetylation of Slug at lysines 166 and 211. Analysis with acetylation-specific antibodies revealed that Slug is highly acetylated in metastatic breast cancer cells. Notably, Slug acetylation, mediated by CBP at lysines 166 and 211, doubles its half-life and increases its stability. Further, acetylated Slug downregulates the expression of E-cadherin, the epithelial marker, and upregulates the expression of N-cadherin and vimentin, thereby promoting breast cancer cell migration. In conclusion, the present study demonstrates that CBP-mediated Slug acetylation increases its stability, promoting EMT and migration of breast cancer cells.
Similar content being viewed by others
References
Alves, C.C., Carneiro, F., Hoefler, H., and Becker, K.F. (2009). Role of the epithelial-mesenchymal transition regulator Slug in primary human cancers. Front Biosci Volume, 3035–3050.
Assani, G., and Zhou, Y. (2019). Effect of modulation of epithelial-mesenchymal transition regulators Snail1 and Snail2 on cancer cell radiosensitivity by targeting of the cell cycle, cell apoptosis and cell migration/invasion (Review). Oncol Lett 171, 23–30.
Bill, R., and Christofori, G. (2015). The relevance of EMT in breast cancer metastasis: Correlation or causality? FEBS Lett 589, 1577–1587.
Bolós, V., Peinado, H., Pérez-Moreno, M.A., Fraga, M.F., Esteller, M., and Cano, A. (2003). The transcription factor Slug represses E-cadherin expression and induces epithelial to mesenchymal transitions: a comparison with Snail and E47 repressors. J Cell Sci 116, 499–511.
Bray, F., Ferlay, J., Soerjomataram, I., Siegel, R.L., Torre, L.A., and Jemal, A. (2018). Global cancer statistics 2018: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J Clin 68, 394–424.
Cobaleda, C., Pérez-Caro, M., Vicente-Dueñas, C., and Sánchez-García, I. (2007). Function of the zinc-finger transcription factor SNAI2 in cancer and development. Annu Rev Genet 41, 41–61.
Demirkan, B. (2013). The roles of epithelial-to-mesenchymal transition (EMT) and mesenchymal-to-epithelial transition (MET) in breast cancer bone metastasis: potential targets for prevention and treatment. J Clin Med 2, 264–282.
DiMeo, T.A., Anderson, K., Phadke, P., Fan, C., Feng, C., Perou, C.M., Naber, S., and Kuperwasser, C. (2009). A novel lung metastasis signature links Wnt signaling with cancer cell self-renewal and epithelial-mesenchymal transition in basal-like breast cancer. Cancer Res 69, 5364–5373.
Felipe Lima, J., Nofech-Mozes, S., Bayani, J., and Bartlett, J.M.S. (2016). EMT in breast carcinoma—a review. J Clin Med 5, 65.
Ferrari-Amorotti, G., Chiodoni, C., Shen, F., Cattelani, S., Soliera, A.R., Manzotti, G., Grisendi, G., Dominici, M., Rivasi, F., Colombo, M.P., et al. (2014). Suppression of invasion and metastasis of triple-negative breast cancer lines by pharmacological or genetic inhibition of slug activity. Neoplasia 16, 1047–1058.
Ferrari-Amorotti, G., Fragliasso, V., Esteki, R., Prudente, Z., Soliera, A.R., Cattelani, S., Manzotti, G., Grisendi, G., Dominici, M., Pieraccioli, M., et al. (2013). Inhibiting interactions of lysine demethylase LSD1 with snail/slug blocks cancer cell invasion. Cancer Res 73, 235–245.
Gross, K.M., Zhou, W., Breindel, J.L., Ouyang, J., Jin, D.X., Sokol, E.S., Gupta, P.B., Huber, K., Zou, L., and Kuperwasser, C. (2019). Loss of Slug compromises DNA damage repair and accelerates stem cell aging in mammary epithelium. Cell Rep 28, 394–407.e6.
Grzegrzolka, J., Biala, M., Wojtyra, P., Kobierzycki, C., Olbromski, M., Gomulkiewicz, A., Piotrowska, A., Rys, J., Podhorska-Okolow, M., and Dziegiel, P. (2015). Expression of EMT markers SLUG and TWIST in breast cancer. Anticancer Res 357, 3961–3968.
Guo, W., Keckesova, Z., Donaher, J.L., Shibue, T., Tischler, V., Reinhardt, F., Itzkovitz, S., Noske, A., Zürrer-Härdi, U., Bell, G., et al. (2012). Slug and Sox9 cooperatively determine the mammary stem cell state. Cell 148, 1015–1028.
Hajra, K.M., Chen, D.Y.S., and Fearon, E.R. (2002). The SLUG zinc-finger protein represses E-cadherin in breast cancer. Cancer Res 626, 1613–1618.
Hemavathy, K., Ashraf, S.I., and Ip, Y.T. (2000). Snail/Slug family of repressors: slowly going into the fast lane of development and cancer. Gene 257, 1–12.
Hung, P.F., Hong, T.M., Chang, C.C., Hung, C.L., Hsu, Y.L., Chang, Y.L., Wu, C.T., Chang, G.C., Chan, N.L., Yu, S.L., et al. (2019). Hypoxia-induced Slug SUMOylation enhances lung cancer metastasis. J Exp Clin Cancer Res 38, 5–24.
Jiang, Y., Zhao, X., Xiao, Q., Liu, Q., Ding, K., Yu, F., Zhang, R., Zhu, T., and Ge, G. (2014). Snail and Slug mediate tamoxifen resistance in breast cancer cells through activation of EGFR-ERK independent of epithelial-mesenchymal transition. J Mol Cell Biol 6, 352–354.
Kao, S.H., Wang, W.L., Chen, C.Y., Chang, Y.L., Wu, Y.Y., Wang, Y.T., Wang, S.P., Nesvizhskii, A.I., Chen, Y.J., Hong, T.M., et al. (2014). GSK3β controls epithelial-mesenchymal transition and tumor metastasis by CHIP-mediated degradation of Slug. Oncogene 33, 3172–3182.
Karamanou, K., Franchi, M., Vynios, D., and Brézillon, S. (2020). Epithelial-to-mesenchymal transition and invadopodia markers in breast cancer: Lumican a key regulator. Seminars Cancer Biol 62, 125–133.
Lehmann, B.D., Bauer, J.A., Chen, X., Sanders, M.E., Chakravarthy, A.B., Shyr, Y., and Pietenpol, J.A. (2011). Identification of human triple-negative breast cancer subtypes and preclinical models for selection of targeted therapies. J Clin Invest 121, 2750–2767.
Luanpitpong, S., Li, J., Manke, A., Brundage, K., Ellis, E., McLaughlin, S. L., Angsutararux, P., Chanthra, N., Voronkova, M., Chen, Y.C., et al. (2016). SLUG is required for SOX9 stabilization and functions to promote cancer stem cells and metastasis in human lung carcinoma. Oncogene 35, 2824–2833.
Ma, J., Liu, C., Yang, D., Song, J., Zhang, J., Wang, T., Wang, M., Xu, W., Li, X., Ding, S., et al. (2019). C1orf106, an innate immunity activator, is amplified in breast cancer and is required for basal-like/luminal progenitor fate decision. Sci China Life Sci 62, 1229–1242.
Meng, J., Ai, X., Lei, Y., Zhong, W., Qian, B., Qiao, K., Wang, X., Zhou, B., Wang, H., Huai, L., et al. (2019). USP5 promotes epithelial-mesenchymal transition by stabilizing SLUG in hepatocellular carcinoma. Theranostics 9, 573–587.
Mittal, M.K., Singh, K., Misra, S., and Chaudhuri, G. (2011). SLUG-induced elevation of D1 cyclin in breast cancer cells through the inhibition of its ubiquitination. J Biol Chem 286, 469–479.
Molina-Ortiz, P., Villarejo, A., MacPherson, M., Santos, V., Montes, A., Souchelnytskyi, S., Portillo, F., and Cano, A. (2012). Characterization of the SNAG and SLUG domains of Snail2 in the repression of E-cadherin and EMT induction: modulation by serine 4 phosphorylation. PLoS ONE 7, e36132.
Nassour, M., Idoux-Gillet, Y., Selmi, A., Côme, C., Faraldo, M.L.M., Deugnier, M.A., and Savagner, P. (2012). Slug controls stem/progenitor cell growth dynamics during mammary gland morphogenesis. PLoS ONE 7, e53498.
Park, J.J., Park, M.H., Oh, E.H., Soung, N.K., Lee, S.J., Jung, J.K., Lee, O. J., Yun, S.J., Kim, W.J., Shin, E.Y., et al. (2018). The p21-activated kinase 4-Slug transcription factor axis promotes epithelial-mesenchymal transition and worsens prognosis in prostate cancer. Oncogene 37, 5147–5159.
Peinado, H., Olmeda, D., and Cano, A. (2007). Snail, Zeb and bHLH factors in tumour progression: an alliance against the epithelial phenotype? Nat Rev Cancer 7, 415–428.
Perez-Losada, J., Sanchez-Martın, M., Rodrıguez-Garcıa, A., Sanchez, M. L., Orfao, A., Flores, T., and Sanchez-Garcıa, I. (2002). Zinc-finger transcription factor Slug contributes to the function of the stem cell factor c-kit signaling pathway. Blood 100, 1274–1286.
Pérez-Mancera, P.A., González-Herrero, I., Pérez-Caro, M., Gutiérrez-Cianca, N., Flores, T., Gutiérrez-Adán, A., Pintado, B., Sánchez-Martín, M., and Sánchez-García, I. (2005). SLUG in cancer development. Oncogene 24, 3073–3082.
Phillips, S., Prat, A., Sedic, M., Proia, T., Wronski, A., Mazumdar, S., Skibinski, A., Shirley, S.H., Perou, C.M., Gill, G., et al. (2014). Cellstate transitions regulated by SLUG are critical for tissue regeneration and tumor initiation. Stem Cell Rep 2, 633–647.
Shih, J.Y., and Yang, P.C. (2011). The EMT regulator slug and lung carcinogenesis. Carcinogenesis 32, 1299–1304.
Shirley, S.H., Hudson, L.G., He, J., and Kusewitt, D.F. (2010). The skinny on Slug. Mol Carcinog 49, 851–861.
Thiery, J.P. (2002). Epithelial-mesenchymal transitions in tumour progression. Nat Rev Cancer 2, 442–454.
Tien, C.L., Jones, A., Wang, H., Gerigk, M., Nozell, S., and Chang, C. (2015). Snail2/Slug cooperates with Polycomb repressive complex 2 (PRC2) to regulate neural crest development. Development 142, 722–731.
Villarejo, A., Cortés-Cabrera, A., Molina-Ortíz, P., Portillo, F., and Cano, A. (2014). Differential role of Snail1 and Snail2 zinc fingers in E-cadherin repression and epithelial to mesenchymal transition. J Biol Chem 289, 930–941.
Wu, Z.Q., Li, X.Y., Yuexian Hu, C., Ford, M., Kleer, C.G., and Weiss, S.J. (2012). Canonical Wnt signaling regulates Slug activity and links epithelial-mesenchymal transition with epigenetic Breast Cancer 1, Early Onset (BRCA1) repression. Proc Natl Acad Sci USA 109, 16654–16659.
Xu, R., Won, J.Y., Kim, C.H., Kim, D.E., and Yim, H. (2019). Roles of the phosphorylation of transcriptional factors in epithelial-mesenchymal transition. J Oncol 2019, 1–11.
Yan, Y., Li, X.Q., Duan, J.L., Bao, C.J., Cui, Y.N., Su, Z.B., Xu, J.R., Luo, Q., Chen, M., Xie, Y., et al. (2019). Nanosized functional miRNA liposomes and application in the treatment of TNBC by silencing Slug gene. Int J Nanomed Volume 14, 3645–3667.
Yang, G., Wang, H., Feng, M., You, L., Zheng, L., Zhang, T., Cong, L., and Zhao, Y. (2019). Integrated analysis of gene expression and methylation profiles of novel pancreatic cancer cell lines with highly metastatic activity. Sci China Life Sci 62, 791–806.
Ye, X., Tam, W.L., Shibue, T., Kaygusuz, Y., Reinhardt, F., Ng Eaton, E., and Weinberg, R.A. (2015). Distinct EMT programs control normal mammary stem cells and tumour-initiating cells. Nature 525, 256–260.
Ye, Y., Xiao, Y., Wang, W., Yearsley, K., Gao, J.X., Shetuni, B., and Barsky, S.H. (2010). ERα signaling through slug regulates E-cadherin and EMT. Oncogene 29, 1451–1462.
Yin, Y., Zhu, Q., Jiang, T., Fan, L., and Qiu, X. (2019). Targeting histones for degradation in cancer cells as a novel strategy in cancer treatment. Sci China Life Sci 62, 1078–1086.
Zhou, W., Ni, T.K., Wronski, A., Glass, B., Skibinski, A., Beck, A., and Kuperwasser, C. (2016). The SIRT2 deacetylase stabilizes Slug to control malignancy of basal-like breast cancer. Cell Rep 17, 1302–1317.
Acknowledgements
This work was supported by grants from Ministry of Science and Technology of the People’s Republic of China (2016YFC1302103), National Natural Science Foundation of China (81621063, 81730071, 81972616 and 81670626), Natural Science Foundation of Beijing Municipality (7171005), Peking University Medical Sciences Grant (BMU 2018JC004), and the Beijing Natural Science Foundation (7202080).
Author information
Authors and Affiliations
Corresponding authors
Ethics declarations
Compliance and ethics The author(s) declare that they have no conflict of interest.
Supporting Information
Rights and permissions
About this article
Cite this article
Dai, X., Xin, Y., Xu, W. et al. CBP-mediated Slug acetylation stabilizes Slug and promotes EMT and migration of breast cancer cells. Sci. China Life Sci. 64, 563–574 (2021). https://doi.org/10.1007/s11427-020-1736-5
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11427-020-1736-5