Abstract
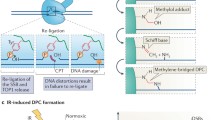
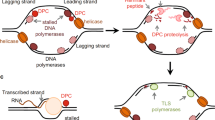
DNA is constantly exposed to a wide array of genotoxic agents, generating a variety of forms of DNA damage. DNA-protein crosslinks (DPCs)—the covalent linkage of proteins with a DNA strand—are one of the most deleterious and understudied forms of DNA damage, posing as steric blockades to transcription and replication. If not properly repaired, these lesions can lead to mutations, genomic instability, and cell death. DPCs can be induced endogenously or through environmental carcinogens and chemotherapeutic agents. Endogenously, DPCs are commonly derived through reactions with aldehydes, as well as through trapping of various enzymatic intermediates onto the DNA. Proteolytic cleavage of the protein moiety of a DPC is a general strategy for removing the lesion. This can be accomplished through a DPC-specific protease and and/or proteasome-mediated degradation. Nucleotide excision repair and homologous recombination are each involved in repairing DPCs, with their respective roles likely dependent on the nature and size of the adduct. The Fanconi anemia pathway may also have a role in processing DPC repair intermediates. In this review, we discuss how these lesions are formed, strategies and mechanisms for their removal, and diseases associated with defective DPC repair.
Similar content being viewed by others
References
Abraham, J., Balbo, S., Crabb, D., and Brooks, P.J. (2011). Alcohol metabolism in human cells causes DNA damage and activates the Fanconi anemia-breast cancer susceptibility (FA-BRCA) DNA damage response network. Alcohol Clin Exp Res 35, 2113–2120.
Baker, D.J., Wuenschell, G., Xia, L., Termini, J., Bates, S.E., Riggs, A.D., and O’Connor, T.R. (2007). Nucleotide excision repair eliminates unique DNA-protein cross-links from mammalian cells. J Biol Chem 282, 22592–22604.
Balakirev, M.Y., Mullally, J.E., Favier, A., Assard, N., Sulpice, E., Lindsey, D.F., Rulina, A.V., Gidrol, X., and Wilkinson, K.D. (2015). Wss1 metalloprotease partners with Cdc48/Doa1 in processing genotoxic SUMO conjugates. eLife 4, e06763.
Bandaru, V., Sunkara, S., Wallace, S.S., and Bond, J.P. (2002). A novel human DNA glycosylase that removes oxidative DNA damage and is homologous to Escherichia coli endonuclease VIII. DNA Repair 1, 517–529.
Barker, S., Weinfeld, M., and Murray, D. (2005). DNA-protein crosslinks: their induction, repair, and biological consequences. Mutat Res 589, 111–135.
Centore, R.C., Yazinski, S.A., Tse, A., and Zou, L. (2012). Spartan/C1orf124, a reader of PCNA ubiquitylation and a regulator of UV-induced DNA damage response. Mol Cell 46, 625–635.
Chaw, Y.F.M., Crane, L.E., Lange, P., and Shapiro, R. (1980). Isolation and identification of cross-links from formaldehyde-treated nucleic acids. Biochemistry 19, 5525–5531.
Cheng, J., Ye, F., Dan, G., Zhao, Y., Wang, B., Zhao, J., Sai, Y., and Zou, Z. (2016). Bifunctional alkylating agent-mediated MGMT-DNA crosslinking and its proteolytic cleavage in 16HBE cells. Toxicol Appl Pharmacol 305, 267–273.
Christman, J.K. (2002). 5-Azacytidine and 5-aza-2′-deoxycytidine as inhibitors of DNA methylation: mechanistic studies and their implications for cancer therapy. Oncogene 21, 5483–5495.
Chválová, K., Brabec, V., and Kaspárková, J. (2007). Mechanism of the formation of DNA-protein cross-links by antitumor cisplatin. Nucleic Acids Res 35, 1812–1821.
Conaway, C.C., Whysner, J., Verna, L.K., and Williams, G.M. (1996). Formaldehyde mechanistic data and risk assessment: endogenous protection from DNA adduct formation. Pharmacol Therapeut 71, 29–55.
Costa, M., Zhitkovich, A., Harris, M., Paustenbach, D., and Gargas, M. (1997). DNA-protein cross-links produced by various chemicals in cultured human lymphoma cells. J Toxicol Environ Health 50, 433–449.
Davis, E.J., Lachaud, C., Appleton, P., Macartney, T.J., Nathke, I., and Rouse, J. (2012). DVC1 (C1orf124) recruits the p97 protein segregase to sites of DNA damage. Nat Struct Mol Biol 19, 1093–1100.
de Graaf, B., Clore, A., and McCullough, A.K. (2009). Cellular pathways for DNA repair and damage tolerance of formaldehyde-induced DNAprotein crosslinks. DNA Repair 8, 1207–1214.
Delabaere, L., Orsi, G.A., Sapey-Triomphe, L., Horard, B., Couble, P., and Loppin, B. (2014). The Spartan ortholog maternal haploid is required for paternal chromosome integrity in the Drosophila zygote. Curr Biol 24, 2281–2287.
Dellarco, V.L. (1988). A mutagenicity assessment of acetaldehyde. Mutat Res 195, 1–20.
DeMott, M.S., Beyret, E., Wong, D., Bales, B.C., Hwang, J.T., Greenberg, M.M., and Demple, B. (2002). Covalent trapping of human DNA polymerase β by the oxidative DNA lesion 2-deoxyribonolactone. J Biol Chem 277, 7637–7640.
Desai, S.D., Liu, L.F., Vazquez-Abad, D., and D’Arpa, P. (1997). Ubiquitindependent destruction of topoisomerase I is stimulated by the antitumor drug camptothecin. J Biol Chem 272, 24159–24164.
Duxin, J.P., Dewar, J.M., Yardimci, H., and Walter, J.C. (2014). Repair of a DNA-protein crosslink by replication-coupled proteolysis. Cell 159, 346–357.
Duxin, J.P., and Walter, J.C. (2015). What is the DNA repair defect underlying Fanconi anemia? Curr Opin Cell Biol 37, 49–60.
Faure, V., Saparbaev, M., Dumy, P., and Constant, J.F. (2005). Action of multiple base excision repair enzymes on the 2′-deoxyribonolactone. Biochem Biophys Res Commun 328, 1188–1195.
Fornace, A.J., Jr., and Seres, D.S. (1982). Repair of trans-Pt(II)diamminedichloride DNA-protein crosslinks in normal and excision-deficient human cells. Mutat Res 94, 277–284.
Fu, Y.V., Yardimci, H., Long, D.T., Ho, T.V., Guainazzi, A., Bermudez, V.P., Hurwitz, J., van Oijen, A., Schärer, O.D., and Walter, J.C. (2011). Selective bypass of a lagging strand roadblock by the eukaryotic replicative DNA helicase. Cell 146, 931–941.
Garaycoechea, J.I., Crossan, G.P., Langevin, F., Daly, M., Arends, M.J., and Patel, K.J. (2012). Genotoxic consequences of endogenous aldehydes on mouse haematopoietic stem cell function. Nature 489, 571–575.
Ghosal, G., Leung, J.W.C., Nair, B.C., Fong, K.W., and Chen, J. (2012). Proliferating cell nuclear antigen (PCNA)-binding protein C1orf124 is a regulator of translesion synthesis. J Biol Chem 287, 34225–34233.
Hashimoto, M., Greenberg, M.M., Kow, Y.W., Hwang, J.T., and Cunningham, R.P. (2001). The 2-deoxyribonolactone lesion produced in DNA by neocarzinostatin and other damaging agents forms cross-links with the base-excision repair enzyme endonuclease III. J Am Chem Soc 123, 3161–3162.
Huang, Y., and Li, L. (2013). DNA crosslinking damage and cancer—a tale of friend and foe. Transl Cancer Res 2, 144–154.
Ide, H., Shoulkamy, M.I., Nakano, T., Miyamoto-Matsubara, M., and Salem, A.M.H. (2011). Repair and biochemical effects of DNA-protein crosslinks. Mutat Res 711, 113–122.
Juhasz, S., Balogh, D., Hajdu, I., Burkovics, P., Villamil, M.A., Zhuang, Z., and Haracska, L. (2012). Characterization of human Spartan/C1orf124, an ubiquitin-PCNA interacting regulator of DNA damage tolerance. Nucleic Acids Res 40, 10795–10808.
Juttermann, R., Li, E., and Jaenisch, R. (1994). Toxicity of 5-aza-2′-deoxycytidine to mammalian cells is mediated primarily by covalent trapping of DNA methyltransferase rather than DNA demethylation. Proc Natl Acad Sci USA 91, 11797–11801.
Karanja, K.K., Lee, E.H., Hendrickson, E.A., and Campbell, J.L. (2014). Preventing over-resection by DNA2 helicase/nuclease suppresses repair defects in Fanconi anemia cells. Cell Cycle 13, 1540–1550.
Kottemann, M.C., and Smogorzewska, A. (2013). Fanconi anaemia and the repair of Watson and Crick DNA crosslinks. Nature 493, 356–363.
Kumari, A., Minko, I.G., Smith, R.L., Lloyd, R.S., and McCullough, A.K. (2010). Modulation of UvrD helicase activity by covalent DNA-protein cross-links. J Biol Chem 285, 21313–21322.
Kuo, H.K., Griffith, J.D., and Kreuzer, K.N. (2007). 5-Azacytidine induced methyltransferase-DNA adducts block DNA replication in vivo. Cancer Res 67, 8248–8254.
Kurtz, A.J., Dodson, M.L., and Lloyd, R.S. (2002). Evidence for multiple imino intermediates and identification of reactive nucleophiles in peptide-catalyzed β-elimination at abasic sites. Biochemistry 41, 7054–7064.
Kurtz, A.J., and Lloyd, R.S. (2003). 1,N2-deoxyguanosine adducts of acrolein, crotonaldehyde, and trans-4-hydroxynonenal cross-link to peptides via schiff base linkage. J Biol Chem 278, 5970–5976.
Kuykendall, J.R., and Bogdanffy, M.S. (1994). Formation and stability of acetaldehyde-induced crosslinks between poly-lysine and poly-deoxyguanosine. Mutat Res 311, 49–56.
Langevin, F., Crossan, G.P., Rosado, I.V., Arends, M.J., and Patel, K.J. (2011). Fancd2 counteracts the toxic effects of naturally produced aldehydes in mice. Nature 475, 53–58.
Lessel, D., Vaz, B., Halder, S., Lockhart, P.J., Marinovic-Terzic, I., Lopez-Mosqueda, J., Philipp, M., Sim, J.C.H., Smith, K.R., Oehler, J., Cabrera, E., Freire, R., Pope, K., Nahid, A., Norris, F., Leventer, R.J., Delatycki, M.B., Barbi, G., von Ameln, S., Högel, J., Degoricija, M., Fertig, R., Burkhalter, M.D., Hofmann, K., Thiele, H., Altmüller, J., Nürnberg, G., Nürnberg, P., Bahlo, M., Martin, G.M., Aalfs, C.M., Oshima, J., Terzic, J., Amor, D.J., Dikic, I., Ramadan, K., and Kubisch, C. (2014). Mutations in SPRTN cause early onset hepatocellular carcinoma, genomic instability and progeroid features. Nat Genet 46, 1239–1244.
Lindahl, T. (1993). Instability and decay of the primary structure of DNA. Nature 362, 709–715.
Loeber, R., Michaelson, E., Fang, Q., Campbell, C., Pegg, A.E., and Tretyakova, N. (2008). Cross-linking of the DNA repair protein O6-alkylguanine DNA alkyltransferase to DNA in the presence of antitumor nitrogen mustards. Chem Res Toxicol 21, 787–795.
Loeber, R., Rajesh, M., Fang, Q., Pegg, A.E., and Tretyakova, N. (2006). Cross-linking of the human DNA repair protein O6-alkylguanine DNA alkyltransferase to DNA in the presence of 1,2,3,4-diepoxybutane. Chem Res Toxicol 19, 645–654.
Loeber, R.L., Michaelson-Richie, E.D., Codreanu, S.G., Liebler, D.C., Campbell, C.R., and Tretyakova, N.Y. (2009). Proteomic analysis of DNA-protein cross-linking by antitumor nitrogen mustards. Chem Res Toxicol 22, 1151–1162.
Lopez-Mosqueda, J., Maddi, K., Prgomet, S., Kalayil, S., Marinovic-Terzic, I., Terzic, J., and Dikic, I. (2016). SPRTN is a mammalian DNA-binding metalloprotease that resolves DNA-protein crosslinks. eLife 5, e21491.
Lorenti Garcia, C., Mechilli, M., Proietti De Santis, L., Schinoppi, A., Katarzyna, K., and Palitti, F. (2009). Relationship between DNA lesions, DNA repair and chromosomal damage induced by acetaldehyde. Mutat Res 662, 3–9.
Lu, K., Collins, L.B., Ru, H., Bermudez, E., and Swenberg, J.A. (2010). Distribution of DNA adducts caused by inhaled formaldehyde is consistent with induction of nasal carcinoma but not leukemia. Toxicol Sci 116, 441–451.
Machida, Y., Kim, M.S., and Machida, Y.J. (2012). Spartan/C1orf124 is important to prevent UV-induced mutagenesis. Cell Cycle 11, 3395–3402.
Mao, Y., Desai, S.D., Ting, C.Y., Hwang, J., and Liu, L.F. (2001). 26 S proteasome- mediated degradation of topoisomerase II cleavable complexes. J Biol Chem 276, 40652–40658.
Mao, Y., Sun, M., Desai, S.D., and Liu, L.F. (2000). SUMO-1 conjugation to topoisomerase I: a possible repair response to topoisomerase-mediated DNA damage. Proc Natl Acad Sci USA 97, 4046–4051.
Marietta, C., Thompson, L.H., Lamerdin, J.E., and Brooks, P.J. (2009). Acetaldehyde stimulates FANCD2 monoubiquitination, H2AX phosphorylation, and BRCA1 phosphorylation in human cells in vitro: implications for alcohol-related carcinogenesis. Mutat Res 664, 77–83.
Maskey, R.S., Kim, M.S., Baker, D.J., Childs, B., Malureanu, L.A., Jeganathan, K.B., Machida, Y., van Deursen, J.M., and Machida, Y.J. (2014). Spartan deficiency causes genomic instability and progeroid phenotypes. Nat Commun 5, 5744.
Mechilli, M., Schinoppi, A., Kobos, K., Natarajan, A.T., and Palitti, F. (2008). DNA repair deficiency and acetaldehyde-induced chromosomal alterations in CHO cells. Mutagenesis 23, 51–56.
Minko, I.G., Kozekov, I.D., Kozekova, A., Harris, T.M., Rizzo, C.J., and Lloyd, R.S. (2008). Mutagenic potential of DNA-peptide crosslinks mediated by acrolein-derived DNA adducts. Mutat Res 637, 161–172.
Minko, I.G., Kurtz, A.J., Croteau, D.L., Van Houten, B., Harris, T.M., and Lloyd, R.S. (2005). Initiation of repair of DNA-polypeptide cross-links by the UvrABC nuclease. Biochemistry 44, 3000–3009.
Minko, I.G., Zou, Y., and Lloyd, R.S. (2002). Incision of DNA-protein crosslinks by UvrABC nuclease suggests a potential repair pathway involving nucleotide excision repair. Proc Natl Acad Sci USA 99, 1905–1909.
Monticello, T.M., Swenberg, J.A., Gross, E.A., Leininger, J.R., Kimbell, J.S., Seilkop, S., Starr, T.B., Gibson, J.E., and Morgan, K.T. (1996). Correlation of regional and nonlinear formaldehyde-induced nasal cancer with proliferating populations of cells. Cancer Res 56, 1012–1022.
Mosbech, A., Gibbs-Seymour, I., Kagias, K., Thorslund, T., Beli, P., Povlsen, L., Nielsen, S.V., Smedegaard, S., Sedgwick, G., Lukas, C., Hartmann-Petersen, R., Lukas, J., Choudhary, C., Pocock, R., Bekker-Jensen, S., and Mailand, N. (2012). DVC1 (C1orf124) is a DNA damage-targeting p97 adaptor that promotes ubiquitin-dependent responses to replication blocks. Nat Struct Mol Biol 19, 1084–1092.
Nakano, T., Katafuchi, A., Matsubara, M., Terato, H., Tsuboi, T., Masuda, T., Tatsumoto, T., Pack, S.P., Makino, K., Croteau, D.L., Van Houten, B., Iijima, K., Tauchi, H., and Ide, H. (2009). Homologous recombination but not nucleotide excision repair plays a pivotal role in tolerance of DNA-protein cross-links in mammalian cells. J Biol Chem 284, 27065–27076.
Nakano, T., Miyamoto-Matsubara, M., Shoulkamy, M.I., Salem, A.M.H., Pack, S.P., Ishimi, Y., and Ide, H. (2013). Translocation and stability of replicative DNA helicases upon encountering DNA-protein cross-links. J Biol Chem 288, 4649–4658.
Nakano, T., Morishita, S., Katafuchi, A., Matsubara, M., Horikawa, Y., Terato, H., Salem, A.M.H., Izumi, S., Pack, S.P., Makino, K., and Ide, H. (2007). Nucleotide excision repair and homologous recombination systems commit differentially to the repair of DNA-protein crosslinks. Mol Cell 28, 147–158.
Nakano, T., Ouchi, R., Kawazoe, J., Pack, S.P., Makino, K., and Ide, H. (2012). T7 RNA polymerases backed up by covalently trapped proteins catalyze highly error prone transcription. J Biol Chem 287, 6562–6572.
Noguchi, C., Grothusen, G., Anandarajan, V., Martínez-Lage García, M., Terlecky, D., Corzo, K., Tanaka, K., Nakagawa, H., and Noguchi, E. (2017). Genetic controls of DNA damage avoidance in response to acetaldehyde in fission yeast. Cell Cycle 16, 45–58.
Orta, M.L., Calderón-Montaño, J.M., Domínguez, I., Pastor, N., Burgos-Morón, E., López-Lázaro, M., Cortés, F., Mateos, S., and Helleday, T. (2013). 5-Aza-2′-deoxycytidine causes replication lesions that require Fanconi anemia-dependent homologous recombination for repair. Nucleic Acids Res 41, 5827–5836.
Orta, M.L., Höglund, A., Calderón-Montaño, J.M., Domínguez, I., Burgos-Morón, E., Visnes, T., Pastor, N., Ström, C., López-lázaro, M., and Helleday, T. (2014). The PARP inhibitor olaparib disrupts base excision repair of 5-aza-2′-deoxycytidine lesions. Nucleic Acids Res 42, 9108–9120.
Ortega-Atienza, S., Green, S.E., and Zhitkovich, A. (2015). Proteasome activity is important for replication recovery, CHK1 phosphorylation and prevention of G2 arrest after low-dose formaldehyde. Toxicol Appl Pharmacol 286, 135–141.
Palii, S.S., Van Emburgh, B.O., Sankpal, U.T., Brown, K.D., and Robertson, K.D. (2008). DNA methylation inhibitor 5-aza-2′-deoxycytidine induces reversible genome-wide DNA damage that is distinctly influenced by DNA methyltransferases 1 and 3B. Mol Cell Biol 28, 752–771.
Pande, P., Ji, S., Mukherjee, S., Schärer, O.D., Tretyakova, N.Y., and Basu, A.K. (2017). Mutagenicity of a model DNA-peptide cross-link in human cells: roles of translesion synthesis DNA polymerases. Chem Res Toxicol 30, 669–677.
Pommier, Y., Barcelo, J.M., Rao, V.A., Sordet, O., Jobson, A.G., Thibaut, L., Miao, Z.H., Seiler, J.A., Zhang, H., Marchand, C., Agama, K., Nitiss, J.L., and Redon, C. (2006). Repair of topoisomerase I-mediated DNA damage. Prog Nucleic Acid Res Mol Biol 81, 179–229.
Pouliot, J.J., Yao, K.C., Robertson, C.A., and Nash, H.A. (1999). Yeast gene for a Tyr-DNA phosphodiesterase that repairs topoisomerase I complexes. Science 286, 552–555.
Pourquier, P., Ueng, L.M., Kohlhagen, G., Mazumder, A., Gupta, M., Kohn, K.W., and Pommier, Y. (1997). Effects of uracil incorporation, DNA mismatches, and abasic sites on cleavage and religation activities of mammalian topoisomerase I. J Biol Chem 272, 7792–7796.
Prasad, R., Horton, J.K., Chastain, P.D., Gassman, N.R., Freudenthal, B.D., Hou, E.W., and Wilson, S.H. (2014). Suicidal cross-linking of PARP-1 to AP site intermediates in cells undergoing base excision repair. Nucleic Acids Res 42, 6337–6351.
Psakhye, I., and Jentsch, S. (2012). Protein group modification and synergy in the SUMO pathway as exemplified in DNA repair. Cell 151, 807–820.
Quievryn, G., and Zhitkovich, A. (2000). Loss of DNA-protein crosslinks from formaldehyde-exposed cells occurs through spontaneous hydrolysis and an active repair process linked to proteosome function. Carcinogenesis 21, 1573–1580.
Quiñones, J.L., Thapar, U., Yu, K., Fang, Q., Sobol, R.W., and Demple, B. (2015). Enzyme mechanism-based, oxidative DNA-protein cross-links formed with DNA polymerase β in vivo. Proc Natl Acad Sci USA 112, 8602–8607.
Reardon, J.T., Cheng, Y., and Sancar, A. (2006). Repair of DNA-protein cross-links in mammalian cells. Cell Cycle 5, 1366–1370.
Reardon, J.T., and Sancar, A. (2006). Repair of DNA-polypeptide crosslinks by human excision nuclease. Proc Natl Acad Sci USA 103, 4056–4061.
Ridpath, J.R., Nakamura, A., Tano, K., Luke, A.M., Sonoda, E., Arakawa, H., Buerstedde, J.M., Gillespie, D.A.F., Sale, J.E., Yamazoe, M., Bishop, D.K., Takata, M., Takeda, S., Watanabe, M., Swenberg, J.A., and Nakamura, J. (2007). Cells deficient in the FANC/BRCA pathway are hypersensitive to plasma levels of formaldehyde. Cancer Res 67, 11117–11122.
Rosado, I.V., Langevin, F., Crossan, G.P., Takata, M., and Patel, K.J. (2011). Formaldehyde catabolism is essential in cells deficient for the Fanconi anemia DNA-repair pathway. Nat Struct Mol Biol 18, 1432–1434.
Ruijs, M.W., van Andel, R.N., Oshima, J., Madan, K., Nieuwint, A.W., and Aalfs, C.M. (2003). Atypical progeroid syndrome: an unknown helicase gene defect? Am J Med GenetPart A 116A, 295–299.
Santi, D.V., Garrett, C.E., and Barr, P.J. (1983). On the mechanism of inhibition of DNA-cytosine methyltransferases by cytosine analogs. Cell 33, 9–10.
Schwilk, E., Zhang, L., Smith, M.T., Smith, A.H., and Steinmaus, C. (2010). Formaldehyde and leukemia: an updated meta-analysis and evaluation of bias. J Occupat Environ Med 52, 878–886.
Sczepanski, J.T., Wong, R.S., McKnight, J.N., Bowman, G.D., and Greenberg, M.M. (2010). Rapid DNA-protein cross-linking and strand scission by an abasic site in a nucleosome core particle. Proc Natl Acad Sci USA 107, 22475–22480.
Solomon, M.J., and Varshavsky, A. (1985). Formaldehyde-mediated DNAprotein crosslinking: a probe for in vivo chromatin structures. Proc Natl Acad Sci USA 82, 6470–6474.
Speit, G., Schutz, P., and Merk, O. (2000). Induction and repair of formaldehyde-induced DNA-protein crosslinks in repair-deficient human cell lines. Mutagenesis 15, 85–90.
Stingele, J., Bellelli, R., Alte, F., Hewitt, G., Sarek, G., Maslen, S.L., Tsutakawa, S.E., Borg, A., Kjær, S., Tainer, J.A., Skehel, J.M., Groll, M., and Boulton, S.J. (2016). Mechanism and regulation of DNA-protein crosslink repair by the DNA-dependent metalloprotease SPRTN. Mol Cell 64, 688–703.
Stingele, J., and Jentsch, S. (2015). DNA-protein crosslink repair. Nat Rev Mol Cell Biol 16, 455–460.
Stingele, J., Schwarz, M.S., Bloemeke, N., Wolf, P.G., and Jentsch, S. (2014). A DNA-dependent protease involved in DNA-protein crosslink repair. Cell 158, 327–338.
Sung, J.S., DeMott, M.S., and Demple, B. (2005). Long-patch base excision DNA repair of 2-deoxyribonolactone prevents the formation of DNA-protein cross-links with DNA polymerase β. J Biol Chem 280, 39095–39103.
Swenberg, J.A., Lu, K., Moeller, B.C., Gao, L., Upton, P.B., Nakamura, J., and Starr, T.B. (2011). Endogenous versus exogenous DNA adducts: their role in carcinogenesis, epidemiology, and risk assessment. Toxicol Sci 120, S130–S145.
Trewick, S.C., Henshaw, T.F., Hausinger, R.P., Lindahl, T., and Sedgwick, B. (2002). Oxidative demethylation by Escherichia coli AlkB directly reverts DNA base damage. Nature 419, 174–178.
Vasiliou, V., Pappa, A., and Estey, T. (2004). Role of human aldehyde dehydrogenases in endobiotic and xenobiotic metabolism. Drug Metab Rev 36, 279–299.
Vaz, B., Popovic, M., Newman, J.A., Fielden, J., Aitkenhead, H., Halder, S., Singh, A.N., Vendrell, I., Fischer, R., Torrecilla, I., Drobnitzky, N., Freire, R., Amor, D.J., Lockhart, P.J., Kessler, B.M., McKenna, G.W., Gileadi, O., and Ramadan, K. (2016). Metalloprotease SPRTN/DVC1 orchestrates replication-coupled DNA-protein crosslink repair. Mol Cell 64, 704–719.
Voitkun, V., Zhitkovich, A., and Costa, M. (1998). Cr(III)-mediated crosslinks of glutathione or amino acids to the DNA phosphate backbone are mutagenic in human cells. Nucleic Acids Res 26, 2024–2030.
Walport, L.J., Hopkinson, R.J., and Schofield, C.J. (2012). Mechanisms of human histone and nucleic acid demethylases. Curr Opin Chem Biol 16, 525–534.
Wickramaratne, S., Ji, S., Mukherjee, S., Su, Y., Pence, M.G., Lior-Hoffmann, L., Fu, I., Broyde, S., Guengerich, F.P., Distefano, M., Schärer, O.D., Sham, Y.Y., and Tretyakova, N. (2016). Bypass of DNA-protein cross-links conjugated to the 7-deazaguanine position of DNA by translesion synthesis polymerases. J Biol Chem 291, 23589–23603.
Yeo, J.E., Wickramaratne, S., Khatwani, S., Wang, Y.C., Vervacke, J., Distefano, M.D., and Tretyakova, N.Y. (2014). Synthesis of site-specific DNA-protein conjugates and their effects on DNA replication. ACS Chem Biol 9, 1860–1868.
Zecevic, A., Hagan, E., Reynolds, M., Poage, G., Johnston, T., and Zhitkovich, A. (2010). XPA impacts formation but not proteasome- sensitive repair of DNA-protein cross-links induced by chromate. Mutagenesis 25, 381–388.
Zhang, L., Steinmaus, C., Eastmond, D.A., Xin, X.K., and Smith, M.T. (2009). Formaldehyde exposure and leukemia: a new meta-analysis and potential mechanisms. Mutat Res 681, 150–168.
Zhitkovich, A., Voitkun, V., and Costa, M. (1995). Glutathione and free amino acids form stable complexes with DNA following exposure of intact mammalian cells to chromate. Carcinogenesis 16, 907–913.
Acknowledgements
We thank Erica Lynn for helpful comments on the manuscript. This work was supported by the National Institutes of Health (CA179441, CA193124-Project 3 to Lei Li) and the Olive Stringer Endowed Professorship (Lei Li).
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Klages-Mundt, N.L., Li, L. Formation and repair of DNA-protein crosslink damage. Sci. China Life Sci. 60, 1065–1076 (2017). https://doi.org/10.1007/s11427-017-9183-4
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11427-017-9183-4