Abstract
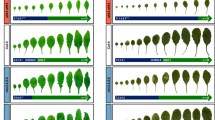
Plants undergo a series of developmental transitions during their life cycle. After seed germination, plants pass through two distinct phases: the vegetative phase in which leaves are produced and the reproductive phase in which flowering occurs. Based on the reproductive competence and morphological changes, the vegetative phase can be further divided into juvenile and adult phases. Here, we demonstrate that the difference between juvenile and adult phase of Nicotiana tabacum is characterized by the changes in leaf size, leaf shape as well as the number of leaf epidermal hairs (trichomes). We further show that miR156, an age-regulated microRNA, regulates juvenile-to-adult phase transition in N. tabacum. Overexpression of miR156 results in delayed juvenile-to-adult transition and flowering. Together, our results support an evolutionarily conserved role of miR156 in plant developmental transitions.
Article PDF
Similar content being viewed by others
Avoid common mistakes on your manuscript.
References
Baurle I, Dean C. The timing of developmental transitions in plants. Cell, 2006, 125: 655–664
Srikanth A, Schmid M. Regulation of flowering time: all roads lead to Rome. Cell Mol Life Sci, 2011, 68: 2013–2037
Andres F, Coupland G. The genetic basis of flowering responses to seasonal cues. Nat Rev Genet, 2012, 13: 627–639
Amasino RM, Michaels SD. The timing of flowering. Plant Physiol, 2010, 154: 516–520
Kobayashi Y, Weigel D. Move on up, it’s time for change-mobile signals controlling photoperiod-dependent flowering. Genes Dev, 2007, 21: 2371–2384
Poethig RS. Phase change and the regulation of developmental timing in plants. Science, 2003, 301: 334–336
Poethig RS. Vegetative phase change and shoot maturation in plants. Curr Top Dev Biol, 2013, 105: 125–152
Huijser P, Schmid M. The control of developmental phase transitions in plants. Development, 2011, 138: 4117–4129
Rogers K, Chen X. Biogenesis, turnover, and mode of action of plant microRNAs. Plant Cell, 2013, 25: 2383–2399
Bartel DP. MicroRNAs: target recognition and regulatory functions. 2009. Cell, 136: 215–233
Zhao T, Li G, Mi S, Li S, Hannon GJ, Wang XJ, Qi Y. A complex system of small RNAs in the unicellular green alga Chlamydomonas reinhardtii. Genes Dev, 2007, 21: 1190–1203
Molnar A, Schwach F, Studholme DJ, Thuenemann EC, Baulcombe DC. miRNAs control gene expression in the single-cell alga Chlamydomonas reinhardtii. Nature, 2007, 447: 1126–1129
Li J, Wu Y, Qi Y. MicroRNAs in a multicellular green alga Volvox carteri. Sci China Life Sci, 2014, 57: 36–45
Poethig RS. Small RNAs and developmental timing in plants. Curr Opin Genet Dev, 2009, 19: 374–378
Poethig RS. The past, present, and future of vegetative phase change. Plant Physiol, 2010, 154: 541–544
Chen X, Zhang Z, Liu D, Zhang K, Li A, Mao L. SQUAMOSA promoter-binding protein-like transcription factors: star players for plant growth and development. J Integr Plant Biol, 2010, 52: 946–951
Wang Y, Wu F, Bai J, He Y. BrpSPL9 (Brassica rapa ssp. pekinensis SPL9) controls the earliness of heading time in Chinese cabbage. Plant Biotechnol J, 2014, 12: 312–321
Wu G, Park MY, Conway SR, Wang JW, Weigel D, Poethig RS. The sequential action of miR156 and miR172 regulates developmental timing in Arabidopsis. Cell, 2009, 138: 750–759
Zhou CM, Zhang TQ, Wang X, Yu S, Lian H, Tang H, Feng ZY, Zozomova-Lihova J, Wang JW. Molecular basis of age-dependent vernalization in Cardamine flexuosa. Science, 2013, 340: 1097–1100
Wang JW, Park MY, Wang LJ, Koo Y, Chen XY, Weigel D, Poethig RS. miRNA control of vegetative phase change in trees. PLoS Genet, 2011, 7: e1002012
Bergonzi S, Albani MC, Ver Loren van Themaat E, Nordstrom KJ, Wang R, Schneeberger K, Moerland PD, Coupland G. Mechanisms of age-dependent response to winter temperature in perennial flowering of Arabis alpina. Science, 2013, 340: 1094–1097
Chuck G, Cigan AM, Saeteurn K, Hake S. The heterochronic maize mutant Corngrass1 results from overexpression of a tandem microRNA. Nat Genet, 2007, 39: 544–549
Eviatar-Ribak T, Shalit-Kaneh A, Chappell-Maor L, Amsellem Z, Eshed Y, Lifschitz E. A cytokinin-activating enzyme promotes tuber formation in tomato. Curr Biol, 2013, 23: 1057–1064
Wang JW, Czech B, Weigel D. miR156-regulated SPL transcription factors define an endogenous flowering pathway in Arabidopsis thaliana. Cell, 2009, 138: 738–749
Wu G, Poethig RS. Poethig, Temporal regulation of shoot development in Arabidopsis thaliana by miR156 and its target SPL3. Development, 2006, 133: 3539–3547
Yu S, Cao L, Zhou CM, Zhang TQ, Lian H, Sun Y, Wu JQ, Huang JR, Wang GD, Wang JW. Sugar is an endogenous cue for juvenile-to-adult phase transition in plants. eLIFE, 2013. 2: e00269
Yang L, Xu M, Koo Y, He J, Poethig RS. Sugar promotes vegetative phase change in Arabidopsis thaliana by repressing the expression of MIR156A and MIR156C. elife, 2013, 2: e00260
Xie K, Shen J, Hou X, Yao J, Li X, Xiao J, Xiong L. Gradual increase of miR156 regulates temporal expression changes of numerous genes during leaf development in rice. Plant Physiol, 2012, 158: 1382–1394
Xie K, Wu C, Xiong L. Genomic organization, differential expression, and interaction of SQUAMOSA promoter-binding-like transcription factors and microRNA156 in rice. Plant Physiol, 2006, 142: 280–293
Cardon G, Hohmann S, Klein J, Nettesheim K, Saedler H, Huijser P. Molecular characterisation of the Arabidopsis SBP-box genes. Gene, 1999, 237: 91–104
Xing S, Salinas M, Hohmann S, Berndtgen R, Huijser P. miR156-targeted and nontargeted SBP-box transcription factors act in concert to secure male fertility in Arabidopsis. Plant Cell, 2010, 22: 3935–3950
Usami T, Horiguchi G, Yano S, Tsukaya H. The more and smaller cells mutants of Arabidopsis thaliana identify novel roles for SQUAMOSA PROMOTER BINDING PROTEIN-LIKE genes in the control of heteroblasty. Development, 2009, 136: 955–964
Schwarz S, Grande AV, Bujdoso N, Saedler H, Huijser P. The microRNA regulated SBP-box genes SPL9 and SPL15 control shoot maturation in Arabidopsis. Plant Mol Biol, 2008, 67: 183–195
Rubio-Somoza I, Zhou CM, Confraria A, Martinho C, von Born P, Baena-Gonzalez E, Wang JW, Weigel D. Temporal control of leaf complexity by miRNA-regulated licensing of protein complexes. Curr Biol, 2014, 24: 2714–2719
Jung JH, Ju Y, Seo PJ, Lee JH, Park CM. The SOC1-SPL module integrates photoperiod and gibberellic acid signals to control flowering time in Arabidopsis. Plant J, 2011, 69: 577–588
Ni Z, Kim ED, Ha M, Lackey E, Liu J, Zhang Y, Sun Q, Chen ZJ. Altered circadian rhythms regulate growth vigour in hybrids and allopolyploids. Nature, 2009, 457: 327–331
Thompson JD, Gibson TJ, Plewniak F, Jeanmougin F, Higgins DG. The CLUSTAL X windows interface: flexible strategies for multiple sequence alignment aided by quality analysis tools. Nucleic Acids Res, 1997, 25: 4876–4882
Huson DH. SplitsTree: analyzing and visualizing evolutionary data. Bioinformatics, 1998, 14: 68–73
Balkunde R, Pesch M, Hulskamp M. Trichome patterning in Arabidopsis thaliana from genetic to molecular models. Curr Top Dev Biol, 2010, 91: 299–321
Telfer A, Bollman KM, Poethig RS. Phase change and the regulation of trichome distribution in Arabidopsis thaliana. Development, 1997, 124: 645–654
Shepherd RW, Bass WT, Houtz RL, Wagner GJ. Phylloplanins of tobacco are defensive proteins deployed on aerial surfaces by short glandular trichomes. Plant Cell, 2005, 17: 1851–1861
Wagner GJ. Secreting glandular trichomes: more than just hairs. Plant Physiol, 1991, 96: 675–679
Salinas M, Xing S, Hohmann S, Berndtgen R, Huijser P. Genomic organization, phylogenetic comparison and differential expression of the SBP-box family of transcription factors in tomato. Planta, 2012, 235: 1171–1184
Franco-Zorrilla JM, Valli A, Todesco M, Mateos I, Puga MI, Rubio-Somoza I, Leyva A, Weigel D, Garcia JA, Paz-Ares J. Target mimicry provides a new mechanism for regulation of microRNA activity. Nat Genet, 2007, 39: 1033–1037
Jiao Y, Wang Y, Xue D, Wang J, Yan M, Liu G, Dong G, Zeng D, Lu Z, Zhu X, Qian Q, Li J. Regulation of OsSPL14 by OsmiR156 defines ideal plant architecture in rice. Nat Genet, 2010, 42: 541–544
Miura K, Ikeda M, Matsubara A, Song XJ, Ito M, Asano K, Matsuoka M, Kitano H, Ashikari M. OsSPL14 promotes panicle branching and higher grain productivity in rice. Nat Genet, 2010, 42: 545–549
Schwab R, Palatnik JF, Riester M, Schommer C, Schmid M, Weigel D. Specific effects of microRNAs on the plant transcriptome. Dev Cell, 2005, 8: 517–527
Ferreira e Silva GF, Silva EM, Azevedo Mda S, Guivin MA, Ramiro DA, Figueiredo CR, Carrer H, Peres LE, Nogueira FT. microRNA156-targeted SPL/SBP box transcription factors regulate tomato ovary and fruit development. Plant J, 2014, 78: 604–618
Wang JW. Regulation of flowering time by the miR156-mediated age pathway. J Exp Bot, 2014, 65: 4723–4730
Author information
Authors and Affiliations
Corresponding author
Additional information
This article is published with open access at link.springer.com
Electronic supplementary material
Rights and permissions
Open Access This article is distributed under the terms of the Creative Commons Attribution 4.0 International License (https://creativecommons.org/licenses/by/4.0), which permits use, duplication, adaptation, distribution, and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made.
About this article
Cite this article
Zhang, T., Wang, J. & Zhou, C. The role of miR156 in developmental transitions in Nicotiana tabacum. Sci. China Life Sci. 58, 253–260 (2015). https://doi.org/10.1007/s11427-015-4808-5
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11427-015-4808-5