Abstract
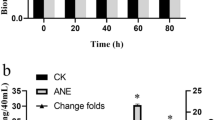
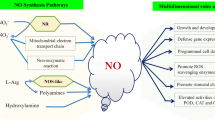
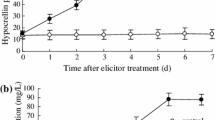
Nitric oxide (NO) has emerged as a key signaling molecule in plant secondary metabolite biosynthesis recently. In order to investigate the molecular basis of NO signaling in elicitor-induced secondary metabolite biosynthesis of plant cells, we determined the contents of NO, salicylic acid (SA), jasmonic acid (JA), and puerarin in Pueraria thomsonii Benth. suspension cells treated with the elicitors prepared from cell walls of Penicillium citrinum. The results showed that the fungal elicitor induced NO burst, SA accumulation and puerarin production of P. thomsonii Benth. cells. The elicitor-induced SA accumulation and puerarin production was suppressed by nitric oxide specific scavenger cPITO, indicating that NO was essential for elicitor-induced SA and puerarin biosynthesis in P. thomsonii Benth. cells. In transgenic NahG P. thomsonii Benth. cells, the fungal elicitor also induced puerarin biosynthesis, NO burst, and JA accumulation, though the SA biosynthesis was impaired. The elicitor-induced JA accumulation in transgenic cells was blocked by cPITO, which suggested that JA acted downstream of NO and its biosynthesis was controlled by NO. External application of NO via its donor sodium nitroprusside (SNP) enhanced puerarin biosynthesis in transgenic NahG P. thomsonii Benth. cells, and the NO-triggered puerarin biosynthesis was suppressed by JA inhibitors IBU and NDGA, which indicated that NO induced puerarin production through a JA-dependent signal pathway in the transgenic cells. Exogenous application of SA suppressed the elicitor-induced JA biosynthesis and reversed the inhibition of IBU and NDGA on elicitor-induced puerarin accumulation in transgenic cells, which indicated that SA inhibited JA biosynthesis in the cells and that SA might be used as a substitute for JA to mediate the elicitor-and NO-induced puerarin biosynthesis. It was, therefore, concluded that NO might mediate the elicitor-induced puerarin biosynthesis through SA-and JA-dependent signal pathways in wildtype P. thomsonii Benth. cells and transgenic NahG cells respectively.
Similar content being viewed by others
References
Verpoorte R, Alfermann A W. Metabolic Engineering of Plant Secondary Metabolism. Dordrecht (The Netherlands): Kluwer Academic Publishers, 2000. 1–29
Roberts S C, Shuler M L. Large-scale plant cell culture. Curr Op Biotechnol, 1997, 8: 154–159
Yu S W, Ou-Yang G C. The material basis of plant pathogenic resistance. In: Yu S W, Tang Z C, eds. Plant Physiology and Molecular Biology (in Chinese). Beijing: Science Press, 1999. 770–783
Ebel J, Scheel D. Elicitor recognition and signal transduction. In: Boller T, Meins F, eds, Genes Involved in Plant Defense. New York: Springer-Verlag, 1992. 183–205
Ciddi V, Srinivasan V, Shuler M L. Elicitation of Taxus sp. cell cultures for production of taxol. Biotechnol Lett, 1995, 17: 1343–1346
Li C, Yuan Y J, Ma Z H, Hu Z D. Changes of physiological state of suspension cultures of Taxus chinensis var. mairei induced by oligosarchride. J Chem Indus Engineer (in Chinese), 2002, 53(11): 1133–1138
Dietrich A, Mayer J E, Hahlbrock K. Fungal elicitor triggers rapid, transient, and specific protein phosphorylation in parsley cell suspension cultures. J Biol Chem, 1990, 265: 6360–6368
Nürnberger T, Colling C, Hahlbrock K, Jabs T, Renelt A, Sacks W R, Scheel D. Perception and transduction of an elicitor signal in cultured parsley cells. Biochem Soc Symp, 1994, 60: 173–182
Baker C J, Orlandi E W. Active oxygen in plant pathogenesis. Annu Rev Phytopathol, 1995, 33: 299–321
Neill S J, Desikan R, Hancock J T. Nitric oxide signaling in plants. New Phytologist, 2003, 159: 11–22
Morot-Gaudry-Talarmain Y, Rockel P, Moureaux T, Quillere I, Leydecker M T, Kaiser W M, Morot-Gaudry J F. Nitrite accumulation and nitric oxide emission in relation to cellular signaling in nitrite reductase antisense plants. Planta, 2002, 215: 708–715
Beligni M V, Lamattina L, Nitric oxide stimulates seed germination and deetiolation, and inhibits hypocotyls elongation, three light-inducible responses in plants. Planta, 2000, 210: 215–222
Delledonne M, Zeier J, Marocco A, Lamb C. Signal interaction between nitric oxide and reactive oxygen intermediates in the plant hypersensitive disease resistance response. Proc Natl Acad Sci USA, 2001, 98: 13454–13459
Modolo L V, Cunha F Q, Braga M R, Salgado I. Nitric oxide synthase-mediated phytoalexin accumulation in soybean cotyledons in response to the Diaporthe phaseolorum f. sp. meridionalis elicitor. Plant Physiol, 2002, 130: 1288–1297
Durner J, Wendehenne D, Klessig D F. Defense gene induction in tobacco by nitric oxide, cyclic CMP and cyclic ADP-ribose. Proc Natl Acad Sci USA, 1998, 95: 10328–10333
Xu M J, Dong J, Zhu M. Effect of nitric oxide on catharanthine production and growth of Catharanthus roseus suspension cells. Biotechol Bioeng, 2005, 89(3): 367–371
Xu M J, Dong J F, Zhu M Y. Involvement of NO in elicitor-induced PAL activation and Taxol synthesis stimulation of Taxus chinensis suspension cells. Chin Sci Bull, 2004, 49(10): 1038–1043
Xu M J, Dong J. Elicitor-induced nitric oxide burst is essential for triggering catharanthine synthesis in Catharanthus roseus suspension cells. Appl Microbiol Biotechnol, 2005, 67(1): 40–44
Kechum R E B. The kinetics of Taxol accumulation in cell suspension cultures of Taxus following elicitation with methyl jasmonate. Biotechol Bioeng, 1999, 62(1): 97–105
Creelman R A, Mullet J E. Biosynthesis and action of jasmonates in plants. Annu Rev Plant Mol Biol, 1997, 48: 355–381
Ellard-Ivey M, Douglas C J. Role of jasmonates in the elicitor-and wound-inducible expression of defense genes in parsley and transgenic tobacco. Plant Physiol, 1996, 112: 183–192
Menke F L H, Parchmann S, Mueller M J, Kijne J W, Memelink J. Involvement of the Octadecanoid Pathway and Protein Phosphorylation in Fungal Elicitor-Induced Expression of Terpenoid Indole Alkaloid Biosynthetic Genes in Catharanthus roseus. Plant Physiol, 1999, 119: 1289–1296
Nojiri H, Sugimori M, Yamane H, Nishimura Y, Yamada A, Shibuya N, Kodama O, Murofushi N, Omori T. Involvement of jasmonic acid in elicitor-induced phytoalexin production in suspension-cultured rice cells. Plant Physiol, 1996, 110: 387–392
Mueller M J, Brodschelm W, Spannagl E, Zenk M H. Signaling in the elicitation process is mediated through the octadecanoid pathway leading to jasmonic acid. Proc Natl Acad Sci USA, 1993, 90: 7490–7494
Shah J. The salicylic acid loop in plant defense. Curr Opin Plant Biol, 2003, 6: 365–371
Thomma B P, Penninckx I A, Broekaert W F, Cammue B P. The complexity of disease signaling in Arabidopsis. Curr Opin Immunol, 2001, 13: 63–68
Gamborg O L, Miller R A, Ojima K. Nutrient requirements of suspension culture of soybean root cultures. Exp Cell Res, 1968, 50: 151–158
Zhang C H, Mei X G, Liu L, Zhang J. Enhanced paclitaxel production induced by the combination of elicitors in cell suspension cultures of Taxus chinensis. Biotechnol Lett, 2000, 22: 1561–1564
Hu X Y, Neill S J, Cai W M, Tang Z C. NO-mediated hypersensitive responses of rice suspension cultures induced by incompatible elicitor. Chin Sci Bull, 2003, 48(4): 358–363
Delledonne M, Xia Y, Dixon R A. Lamb C. Nitric oxide functions as a secondary signal in plant disease resistance. Nature, 1998, 394: 585–588
Alami I, Jouy N, Clervet A. The lipoxygenase pathway is involved in elicitor-induced phytoalexin accumulation in plane tree (Platanus acerifolia) cell-suspension cultures. J Phytopathol, 1999, 147: 515–519
Meuwly P, Metraux J P. Ortho-anisic acid as internal standard for the simultaneous quantitation of salicylic acid and its putative biosynthetic precursors in cucumber leaves. Anal Biochem, 1993, 214: 500–505
Fournier J, Pouenat M L, Rickaner M. Purification and characterization of elicitor-induced lipoxygenase in tobacco cells. Plant J, 1993, 3: 63–70
Bradford M M. A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle fo protein-dye binding. Anal Biochem, 1976, 72: 248–254
He J T, Shi Z H, Zhao M P, Chang W B. Determination of Puerarin and Daidzein by reversed phase HPLC. Chin J Analyt Chem, 2004, 32(4): 519–521
Camera L S, Gouzerh G, Dhondt S, Hoffmann L, Fritig B, Legrand M, Heitz T. Metabolic reprogramming in plants innate immunity: The contributions of phenylpropaniod and oxylipin pathways. Immunol Rev, 2004, 198: 267–284
Tabata H. Paclitaxel production by plant-cell-culture technology. Adv Biochem Eng Biotechnol, 2004, 87: 1–23
Sanchez-Sampedro M A, Fernandez-Tarrago J, Corchete P. Yeast extract and methyl jasmonate-induced silymarin production in cell cultures of Silybum marianum (L.) Gaertn, J Biotechnol, 2005, 119(1): 60–69
Suzuki H, Reddy M S, Naoumkina M, Aziz N, May G D, Huhman D V, Sumner L W, Blount J W, Mendes P, Dixon R A. Methyl jasmonate and yeast elicitor induce differential transcriptional and metabolic re-programming in cell suspension cultures of the model legume Medicago truncatula. Planta, 2005, 220(5): 696–707
Mei X G. Production of Taxol by Taxus chinensis Cell Cultures (in Chinese). Wuhan: Press of the Scientific University of Central China, 2003. 50–89
Yukimune Y, Tabata H, Higashi Y, Hara Y. Methyl jasmonate-induced over production of paclitaxel and baccation III in taxus cell suspension cultures. Nature Biotechnology, 1996, 14: 1129–1132
Huang X, Stettmaier K, Michel C, Hutzler P, Mueller M J M, Durner J. Nitric oxide is induced by wounding and influences jasmonic acid signaling in Arabidopsis thaliana. Planta, 2004, 218(6): 938–946
Kunkel B N, Brooks D M. Cross talk between signaling pathways in pathogen defense. Curr Opin Plant Biol, 2002, 5: 325–331
Creelman R A, Mullet J E. Biosynthesis and action of jasmonates in plants. Annu Rev Plant Physiol Plant Mol Biol, 1997, 48: 355–381
Seo S, Sano H, Ohashi Y. Jasmonic acid in wound signal transduction pathways. Physiol Plant, 1997, 101: 740–745
Spoel S H. NPR1 modulates cross-talk between salicylate-and jasmonate-dependent defense pathways through a novel function in the cytosol. Plant Cell, 2003, 15: 760–770
Gupta V, Willits M G, Glazebrook J. Arabidopsis thaliana EDS4 contributes to salicylic acid (SA)-dependent expression of dsefense responses: Evidence for inhibition of jasmonic acid signaling by SA. Mol Plant-Microbe Interact, 2000, 13: 503–511
Niki T, Mitsuhara I, Seo S, Hidaka H. Antagonistic effect of salicylic acid and jasmonic acid on the expression of pathogenesis-related (PR) protein genes in wounded mature tobacco leaves. Plant Cell Physiol, 1998, 39: 500–507
Ribeiro E A Jr., Cunha F Q, Tamashiro W M, Martins I S. Growth phase-dependent subcellular localization of nitric oxide synthase in maize cells. FEBS Lett, 1999, 445(2–3): 283–286
Foissner I, Wendehenne D, Langebartels C, Durner J. In vivo imaging of an elicitor-induced nitric oxide burst in tobacco. Plant J, 2000, 23(6): 817–824
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Xu, M., Dong, J. & Zhu, M. Nitric oxide mediates the fungal elicitor-induced puerarin biosynthesis in Pueraria thomsonii Benth. suspension cells through a salicylic acid (SA)-dependent and a jasmonic acid (JA)-dependent signal pathway. SCI CHINA SER C 49, 379–389 (2006). https://doi.org/10.1007/s11427-006-2010-5
Received:
Accepted:
Issue Date:
DOI: https://doi.org/10.1007/s11427-006-2010-5