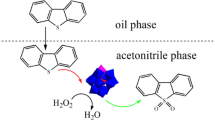
Abstract
It is urgent to develop a new deep desulfurization process of fuels as the environmental pollution increases seriously. In this work, a series of Lewis acidic ionic liquids (ILs) [C 34 MPy]Cl/nZnCl2 (n=1, 1.5, 2, 3) were synthesized and used in extraction and catalytic oxidative desulfurization (ECOD) of the fuels. The effects of the Lewis acidity of ILs, the molar ratio of H2O2/sulfur, temperatures, and different substrates including dibenzothiophene (DBT), benzothiophene (BT) and thiophene (TS), on sulfur removal were investigated. The results indicated that [C 34 MPy]Cl/3ZnCl2 presented near 100% DBT removal of model oil under conditions of 323 K, H2O2/DBT molar ratio 6:1. Kinetics for the removal of DBT, BT and TS by the [C 34 MPy]Cl/3ZnCl2-H2O2 system at 323 K is first-order with the apparent rate constants of 1.1348, 0.2226 and 0.0609 h-1, and the calculated apparent activation energies for DBT, BT and TS were 61.13, 60.66, and 68.14 kJ/mol from 298 to 308 K, respectively. After six cycles of the regenerated [CC 34 MPy]Cl/3ZnCl2, the sulfur removal had a slight decrease. [CC 34 MPy]Cl/3ZnCl2 showed a good desulfurization performance under optimal conditions.
Similar content being viewed by others
References
Di Giuseppe A, Crucianelli M, De Angelis F, Crestini C, Saladino R. Appl Catal B: Environ, 2009, 89: 239–245
Chandra Srivastava V. RSC Adv, 2012, 2: 759–783
Zhu WS, Li HM, Jiang X, Yan YS, Lu JD, Xia JX. Energy Fuels, 2007, 21: 2514–2516
Li FT, Kou CG, Sun ZM, Hao YJ, Liu RH, Zhao DS. J Hazard Mater, 2012, 205–206: 164–170
Campos-Martin JM, Capel-Sanchez MC, Perez-Presas P, Fierro JLG. J Chem Technol Biotechnol, 2010, 85: 879–890
Gao HS, Li YG, Wu Y, Luo MF, Li Q, Xing JM, Liu HZ. Energy Fuels, 2009, 23: 2690–2694
Zhang J, Wang AJ, Li X, Ma XH. J Catal, 2011, 279: 269–275
Nie Y, Li CX, Sun AJ, Meng H, Wang ZH. Energy Fuels, 2006, 20: 2083–2087
He LN, Li HM, Zhu WS, Guo JX, Jiang X, Lu JD, Yan YS. Ind Eng Chem Res, 2008, 47: 6890–6895
Kulkarni PS, Afonso CAM. Green Chem, 2010, 12: 1139–1149
Wang T, Zhao DS, Sun ZM, Li FT, Song YQ, Kou CG. Pet Sci Technol, 2012, 30: 385–392
Lu L, Cheng SF, Gao JB, Gao GH, He MY. Energy Fuels, 2007, 21: 383–384
Xu D, Zhu WS, Li HM, Zhang JT, Zou F, Shi H, Yan YS. Energy Fuels, 2009, 23: 5929–5933
Wang JL, Zhao DS, Li KX. Energy Fuels, 2010, 24: 2527–2529
Francisco M, Arce A, Soto A. Fluid Phase Equilib, 2010, 294: 39–48
Li HM, Zhu WS, Wang Y, Zhang JT, Lu JD, Yan YS. Green Chem, 2009, 11: 810–815
Schmidt R. Energy Fuels, 2008, 22: 1774–1778
Ko NH, Lee JS, Huh ES, Lee H, Jung KD, Kim HS, Cheong M. Energy Fuels, 2008, 22: 1687–1690
Chao Y, Li H, Zhu W, Zhu G, Yan Y. Pet Sci Technol, 2010, 28: 1242–1249
Ding YX, Zhu WS, Li HM, Jiang W, Zhang M, Duan YQ, Chang YH. Green Chem, 2011, 13: 1210–1216
Yu GR, Zhao JJ, Song DD, Asumana C, Zhang XY, Chen XC. Ind Eng Chem Res, 2011, 50: 11690–11697
Nie Y, Gong X, Gao HS, Zhang XP, Zhang SJ. Sci China Chem, 2014, 57: 1766–1773
Dupont J, Suarez PAZ, Umpierre AP, De Souza RF. Catal Lett, 2001, 73: 2–4
Li FT, Liu RH, Wen JH, Zhao DS, Sun ZM, Liu Y. Green Chem, 2009, 11: 883–888
Chen XC, Song DD, Asumana C, Yu GR. J Mol Catal A: Chem, 2012, 359: 8–13
Huang WL, Zhu WS, Li HM, Shi H, Zhu GP, Liu H, Chen GY. Ind Eng Chem Res, 2010, 49: 8998–9003
Zhao DS, Wang JL, Zhou EP. Green Chem, 2007, 9: 1219–1222
Gao HS, Guo C, Xing JM, Liu HZ. Sep Sci Technol, 2012, 47: 325–330
Xu JH, Zhao S, Chen W, Wang M, Song YF. Chem Eur J, 2012, 18: 4775–4781
Zhao DS, Li FT, Liu R, Shan HD. Energy Fuels, 2008, 22: 3065–3069
Ambika SPP, Chauhan SMS. New J Chem, 2012, 36: 650–655
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Nie, Y., Dong, Y., Gao, H. et al. Regulating sulfur removal efficiency of fuels by Lewis acidity of ionic liquids. Sci. China Chem. 59, 526–531 (2016). https://doi.org/10.1007/s11426-016-5563-6
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11426-016-5563-6