Abstract
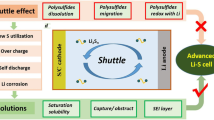
Lithium-sulfur (Li-S) battery is a promising choice for the next generation of high-energy rechargeable batteries, but its application is impeded by the high dissolution of the polysulfides in commonly used organic electrolyte. Room temperature ionic liquids (RTILs) have been considered as appealing candidates for the electrolytes in Li-S batteries. We investigated the effect of cations in RTILs on the electrochemical performance for Li-S batteries. Ex situ investigation of lithium anode for Li-S batteries indicates that during the discharge/charge process the RTIL with N-methyl-N-propylpyrrolidine cations (P13) can effectively suppress the dissolution of the polysulfides, whereas the RTIL with 1-methyl-3-propyl imidazolium cation (PMIM) barely alleviates the shuttling problem. With 0.5 mol L−1 LiTFSI/P13TFSI as the electrolyte of Li-S battery, the ketjen black/sulfur cathode material exhibits high capacity and remarkable cycling stability, which promise the application of the P13-based RTILs in Li-S batteries.
Similar content being viewed by others
References
Armand M, Tarascon JM. Building better batteries. Nature, 2008, 451: 652–657
Gao XP, Yang HX. Multi-electron reaction materials for high energy density batteries. Energe Environ Sci, 2010, 3: 174–189
Guo YG, Hu JS, Wan LJ. Nanostructured materials for electrochemical energy conversion and storage devices. Adv Mater, 2008, 20: 2878–2887
Li H, Wang ZX, Chen LQ, Huang XJ. Research on advanced materials for Li-ion batteries. Adv Mater, 2009, 21: 4593–4607
Luo JY, Cui WJ, He P, Xia YY. Raising the cycling stability of aqueous lithium-ion batteries by eliminating oxygen in the electrolyte. Nat Chem, 2010, 2: 760–765
Wei GZ, Lu X, Ke FS, Huang L, Li JT, Wang ZX, Zhou ZY, Sun SG. Crystal habit-tuned nanoplate material of Li[Li1/3−2x/3NixMn2/3−x/3]O2 for high-rate performance lithium-ion batteries. Adv Mater, 2010, 22: 4364–4367
Yin YX, Xin S, Wan LJ, Li CJ, Guo YG. SnO2 hollow spheres: polymer bead-templated hydrothermal synthesis and their electrochemical properties for lithium storage. Sci China Chem, 2012, 55: 1314–1318
Zhang H, Cao GP, Wang ZY, Yang YS, Shi ZJ, Gu ZN. Carbon nanotube array anodes for high-rate Li-ion batteries. Electrochim Acta, 2010, 55: 2873–2877
Zhang T, Fu LJ, Gao J, Yang LC, Wu YP, Wu HQ. Core-shell Si/C nanocomposite as anode material for lithium ion batteries. Pure Appl Chem, 2006, 78: 1889–1896
Cao AM, Hu JS, Wan LJ. Morphology control and shape evolution in 3D hierarchical superstructures. Sci China Chem, 2012, 55: 2249–2256
Cui GL, Gu L, Zhi LJ, Kaskhedikar N, van Aken PA, Müllen K, Maier J. A germanium-carbon nanocomposite material for lithium batteries. Adv Mater, 2008, 20: 3079–3083
Xin S, Guo YG, Wan LJ. Nanocarbon networks for advanced rechargeable lithium batteries. Acc Chem Res, 2012, 45: 1759–1769
Zhang A, Zheng Z, Cheng F, Tao Z, Chen J. Preparation of Li4Ti5O12 submicrospheres and their application as anode materials of rechargeable lithium-ion batteries. Sci China Chem, 2011, 54: 936–940
Deng X, Liu X, Yan H, Wang D, Wan L. Morphology and modulus evolution of graphite anode in lithium ion battery: an in situ AFM investigation. Sci China Chem, 2014, 57: 178–183
Gao MR, Xu YF, Jiang J, Yu SH. Nanostructured metal chalcogenides: synthesis, modification, and applications in energy conversion and storage devices. Chem Soc Rev, 2013, 42: 2986–3017
Wang ZL, Xu D, Xu JJ, Zhang LL, Zhang XB. Graphene oxide gelderived, free-standing, hierarchically porous carbon for high-capacity and high-rate rechargeable Li-O2 batteries. Adv Funct Mater, 2012, 22: 3699–3705
Bruce PG, Freunberger SA, Hardwick LJ, Tarascon JM. Li-O2 and Li-S batteries with high energy storage. Nat Mater, 2012, 11: 19–29
Xin S, Guo YG, Wan LJ. Electrode materials for lithium secondary batteries with high energy densities. Sci Sinica Chim, 2011, 41: 1–11
Yin YX, Xin S, Guo YG, Wan LJ. Lithium-sulfur batteries: electrochemistry, materials, and prospects. Angew Chem Int Ed, 2013, 52: 13186–13200
Ji XL, Lee KT, Nazar LF. A highly ordered nanostructured carbon-sulphur cathode for lithium-sulphur batteries. Nat Mater, 2009, 8: 500–506
Yang Y, McDowell MT, Jackson A, Cha JJ, Hong SS, Cui Y. New nanostructured Li2S/silicon rechargeable battery with high specific energy. Nano Lett, 2010, 10: 1486–1491
Zhang SS. Liquid electrolyte lithium/sulfur battery: fundamental chemistry, problems, and solutions. J Power Sources, 2013, 231: 153–162
Xin S, Gu L, Zhao NH, Yin YX, Zhou LJ, Guo YG, Wan LJ. Smaller sulfur molecules promise better lithium-sulfur batteries. J Am Chem Soc, 2012, 134: 18510–18513
Xin S, Yin YX, Wan LJ, Guo YG. Encapsulation of sulfur in a hollow porous carbon substrate for superior Li-S batteries with long lifespan. Part Part Syst Char, 2013, 30: 321–325
Ye H, Yin YX, Xin S, Guo YG. Tuning the porous structure of carbon hosts for loading sulfur toward long lifespan cathode materials for Li-S batteries. J Mater Chem A, 2013, 1: 6602–6608
Gao J, Lowe MA, Kiya Y, Abruña HD. Effects of liquid electrolytes on the charge-discharge performance of rechargeable lithium/sulfur batteries: electrochemical and in-situ X-ray absorption spectroscopic studies. J Phy Chem C, 2011, 115: 25132–25137
Ji L, Rao M, Zheng H, Zhang L, Li Y, Duan W, Guo J, Cairns EJ, Zhang Y. Graphene oxide as a sulfur immobilizer in high performance lithium/sulfur cells. J Am Chem Soc, 2011, 133: 18522–18525
Suo L, Hu YS, Li H, Armand M, Chen L. A new class of solvent-in-salt electrolyte for high-energy rechargeable metallic lithium batteries. Nat Commun, 2013, 4: 1–9
Zhang SS. Role of LiNO3 in rechargeable lithium/sulfur battery. Electrochim Acta, 2012, 70: 344–348
Lin Z, Liu ZC, Fu WJ, Dudney NJ, Liang CD. Phosphorous pentasulfide as a novel additive for high-performance lithium-sulfur batteries. Adv Funct Mater, 2013, 23: 1064–1069
Reale P, Fernicola A, Scrosati B. Compatibility of the Py24TFSI-LiTFSI ionic liquid solution with Li4Ti5O12 and LiFePO4 lithium ion battery electrodes. J Power Sources, 2009, 194: 182–189
Wang J, Chew SY, Zhao ZW, Ashraf S, Wexler D, Chen J, Ng SH, Chou SL, Liu HK. Sulfur-mesoporous carbon composites in conjunction with a novel ionic liquid electrolyte for lithium rechargeable batteries. Carbon, 2008, 46: 229–235
Wang L, Byon HR. N-methyl-N-propylpiperidinium bis(trifluoromethanesulfonyl)imide-based organic electrolyte for high performance lithium-sulfur batteries. J Power Sources, 2013, 236: 207–214
Kim S, Jung YJ, Park SJ. Effects of imidazolium salts on discharge performance of rechargeable lithium-sulfur cells containing organic solvent electrolytes. J Power Sources, 2005, 152: 272–277
Kim S, Jung YJ, Park SJ. Effect of imidazolium cation on cycle life characteristics of secondary lithium-sulfur cells using liquid electrolytes. Electrochim Acta, 2007, 52: 2116–2122
Yuan LX, Feng JK, Ai XP, Cao YL, Chen SL, Yang HX. Improved dischargeability and reversibility of sulfur cathode in a novel ionic liquid electrolyte. Electrochem Commun, 2006, 8: 610–614
Park JW, Yamauchi K, Takashima E, Tachikawa N, Ueno K, Dokko K, Watanabe M. Solvent effect of room temperature ionic liquids on electrochemical reactions in lithium-sulfur batteries. J Phy Chem C, 2013, 117: 4431–4440
Author information
Authors and Affiliations
Corresponding authors
Rights and permissions
About this article
Cite this article
Yan, Y., Yin, Y., Guo, Y. et al. Effect of cations in ionic liquids on the electrochemical performance of lithium-sulfur batteries. Sci. China Chem. 57, 1564–1569 (2014). https://doi.org/10.1007/s11426-014-5154-3
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11426-014-5154-3